Redox Biology ( IF 11.4 ) Pub Date : 2020-12-18 , DOI: 10.1016/j.redox.2020.101840 David Siegel 1 , Stephanie Bersie 1 , Peter Harris 1 , Andrea Di Francesco 2 , Michael Armstrong 1 , Nichole Reisdorph 1 , Michel Bernier 2 , Rafael de Cabo 2 , Kristofer Fritz 1 , David Ross 1
|
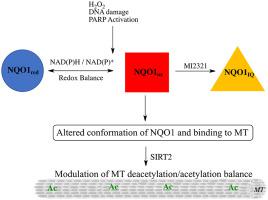
The localization of NQO1 near acetylated microtubules has led to the hypothesis that NQO1 may work in concert with the NAD+-dependent deacetylase SIRT2 to regulate acetyl α-tubulin (K40) levels on microtubules. NQO1 catalyzes the oxidation of NADH to NAD+ and may supplement levels of NAD+ near microtubules to aid SIRT2 deacetylase activity. While HDAC6 has been shown to regulate the majority of microtubule acetylation at K40, SIRT2 is also known to modulate microtubule acetylation (K40) in the perinuclear region. In this study we examined the potential roles NQO1 may play in modulating acetyl α-tubulin levels. Knock-out or knock-down of NQO1 or SIRT2 did not change the levels of acetyl α-tubulin in 16HBE human bronchial epithelial cells and 3T3-L1 fibroblasts; however, treatment with a mechanism-based inhibitor of NQO1 (MI2321) led to a short-lived temporal increase in acetyl α-tubulin levels in both cell lines without impacting the intracellular pools of NADH or NAD+. Inactivation of NQO1 by MI2321 resulted in lower levels of NQO1 immunostaining on microtubules, consistent with redox-dependent changes in NQO1 conformation as evidenced by the use of redox-specific, anti-NQO1 antibodies in immunoprecipitation studies. Given the highly dynamic nature of acetylation-deacetylation reactions at α-tubulin K40 and the crowded protein environment surrounding this site, disruption in the binding of NQO1 to microtubules may temporally disturb the physical interactions of enzymes responsible for maintaining the microtubule acetylome.
中文翻译:

氧化还原介导的 NQO1 构象变化控制与微管的结合和 α-微管蛋白乙酰化
NQO1 在乙酰化微管附近的定位导致了这样的假设,即 NQO1 可能与 NAD +依赖性脱乙酰酶 SIRT2 协同工作以调节微管上的乙酰 α-微管蛋白 (K 40 ) 水平。NQO1 催化 NADH 氧化为 NAD +并可能补充微管附近的 NAD +水平以帮助 SIRT2 脱乙酰酶活性。虽然 HDAC6 已被证明可调节 K 40的大部分微管乙酰化,但已知 SIRT2 可调节微管乙酰化(K 40) 在核周区域。在这项研究中,我们检查了 NQO1 在调节乙酰 α-微管蛋白水平中可能发挥的潜在作用。敲除或敲除 NQO1 或 SIRT2 不会改变 16HBE 人支气管上皮细胞和 3T3-L1 成纤维细胞中乙酰 α-微管蛋白的水平;然而,使用基于机制的 NQO1 抑制剂 (MI2321) 治疗导致两种细胞系中乙酰 α-微管蛋白水平的短暂时间增加,而不影响 NADH 或 NAD +的细胞内池. MI2321 对 NQO1 的灭活导致微管上的 NQO1 免疫染色水平降低,这与在免疫沉淀研究中使用氧化还原特异性抗 NQO1 抗体所证明的 NQO1 构象的氧化还原依赖性变化一致。鉴于 α-微管蛋白 K 40的乙酰化-去乙酰化反应的高度动态性以及该位点周围拥挤的蛋白质环境,NQO1 与微管结合的中断可能会暂时扰乱负责维持微管乙酰组的酶的物理相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号