Colloid and Interface Science Communications ( IF 4.5 ) Pub Date : 2020-12-18 , DOI: 10.1016/j.colcom.2020.100352 Taiki Nishiyama , Kento Sugiura , Kouta Sugikawa , Atsushi Ikeda , Toshihisa Mizuno
|
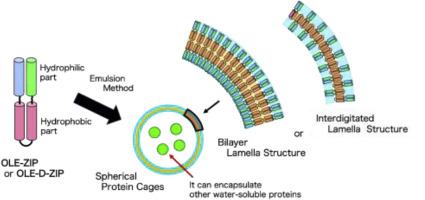
We successfully designed the bilayer-capsule-formable OLE-ZIP proteins by fusing the dimeric coiled coil motif (NZ and CZ) [1] and the hydrophobic domain OLE(56–106) of the sunflower-derived oleosin. In order to improve handling in a buffer, we characterized the fusion protein with thioredoxin, Trx-OLE-ZIP as an alternative. Based on the emulsion method, Trx-OLE-ZIP could successfully form stable liposome-kind bilayer capsules, with empty hollow. Furthermore, it could encapsulate other water-soluble proteins, such as GFP, to the internal hollow with a buffer of inner aqueous phase. By mutating four conserved Thr residues (Thr59, Thr66, Thr97, and Thr104) in the OLE(56–106) domain with Asp residues, we further successfully constructed the pH-sensitive mutant, Trx-OLE-D-ZIP. Under weakly acidic conditions at pH = 5, Trx-OLE-D-ZIP could maintain a spherical cage-morphology. By shifting the solution pH to basic (pH = 9), the protein-cages spontaneously collapsed and released the loaded proteins.
中文翻译:

使用油质蛋白疏水域和亲水二聚体卷曲螺旋的杂合蛋白构建可装载蛋白的蛋白笼
我们通过融合向日葵衍生的油质蛋白的二聚卷曲螺旋基序(NZ和CZ)[1]和疏水域OLE(56-106),成功设计了双层胶囊可形成的OLE-ZIP蛋白。为了改善在缓冲液中的处理,我们用硫氧还蛋白(Trx-OLE-ZIP)作为替代蛋白来表征融合蛋白。基于乳液法,Trx-OLE-ZIP可以成功地形成稳定的脂质体双层胶囊,空洞。此外,它可以用内部水相缓冲液将其他水溶性蛋白(例如GFP)封装到内部空腔中。通过突变四个保守的Thr残基(Thr 59,Thr 66,Thr 97和Thr 104)在带有Asp残基的OLE(56–106)域中,我们进一步成功构建了对pH敏感的突变体Trx-OLE-D-ZIP。在pH = 5的弱酸性条件下,Trx-OLE-D-ZIP可以保持球形的笼形结构。通过将溶液的pH调至碱性(pH = 9),蛋白笼自发塌陷并释放出负载的蛋白。


























 京公网安备 11010802027423号
京公网安备 11010802027423号