当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Remote methylene C(sp3)–H functionalization enabled by organophosphine-catalyzed alkyne isomerization
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-9 , DOI: 10.1039/d0qo01399d De Wang 1, 2, 3, 4, 5 , Zefeng Song 1, 2, 3, 4, 5 , Jianyu Zhang 1, 2, 3, 4, 5 , Tao Xu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-9 , DOI: 10.1039/d0qo01399d De Wang 1, 2, 3, 4, 5 , Zefeng Song 1, 2, 3, 4, 5 , Jianyu Zhang 1, 2, 3, 4, 5 , Tao Xu 1, 2, 3, 4, 5
Affiliation
|
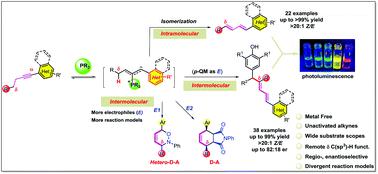
A remote δ methylene C(sp3)–H functionalization catalyzed by an organophosphine through an alkyne isomerization/conjugate addition cascade was discovered. No classical electron-withdrawing group is required to pre-activate the aryl alkyne, which will undergo isomerization to its corresponding diene under only 10 mol% of PMe2Ph with a catalytic proton shuttle. The phosphoryl ylide was postulated as a key intermediate and different electrophiles were used to trap the vinylogous ylide that resulted in a large group of δ C(sp3)–H functionalized products (>60 examples, yields up to 99%) with good diastereoselectivity. Mechanistic studies were carried out and a catalytic cycle was proposed based on deuterium-labeling experiments. The conjugated polyene products were investigated for their fluorescence properties.
中文翻译:

通过有机膦催化的炔烃异构化实现的远程亚甲基C(sp3)–H功能化
通过炔烃异构化/共轭加成级联反应,发现了有机膦催化的远程δ亚甲基C(sp 3)-H官能化。不需要经典的吸电子基团来预活化芳基炔烃,该芳基炔烃将在仅10 mol%的PMe 2 Ph催化下通过质子梭进行异构化为其相应的二烯。假定磷酰叶立德作为关键中间体,并且使用不同的亲电子试剂捕获乙烯基叶立德,从而产生了大量的δC(sp 3)–H功能化产物(> 60个实例,产率高达99%),具有非对映选择性。进行了机理研究,并基于氘标记实验提出了催化循环。研究了共轭多烯产物的荧光性质。
更新日期:2020-12-17
中文翻译:

通过有机膦催化的炔烃异构化实现的远程亚甲基C(sp3)–H功能化
通过炔烃异构化/共轭加成级联反应,发现了有机膦催化的远程δ亚甲基C(sp 3)-H官能化。不需要经典的吸电子基团来预活化芳基炔烃,该芳基炔烃将在仅10 mol%的PMe 2 Ph催化下通过质子梭进行异构化为其相应的二烯。假定磷酰叶立德作为关键中间体,并且使用不同的亲电子试剂捕获乙烯基叶立德,从而产生了大量的δC(sp 3)–H功能化产物(> 60个实例,产率高达99%),具有非对映选择性。进行了机理研究,并基于氘标记实验提出了催化循环。研究了共轭多烯产物的荧光性质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号