当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N ‐Methylated Analogs of hIAPP Fragments 18–22, 23–27, 33–37 Inhibit Aggregation of the Amyloidogenic Core of the Hormone
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2020-12-17 , DOI: 10.1002/cbdv.202000842 Kamil Rozniakowski 1 , Krystian Galecki 2 , Joanna Wietrzyk 3 , Beata Filip-Psurska 3 , Justyna Fraczyk 1 , Zbigniew J Kaminski 1 , Beata Kolesinska 1
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2020-12-17 , DOI: 10.1002/cbdv.202000842 Kamil Rozniakowski 1 , Krystian Galecki 2 , Joanna Wietrzyk 3 , Beata Filip-Psurska 3 , Justyna Fraczyk 1 , Zbigniew J Kaminski 1 , Beata Kolesinska 1
Affiliation
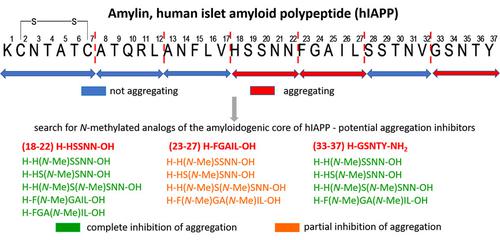
|
Amylin (hIAPP) aggregation leads to the formation of insoluble deposits and is one of the factors in the development of type II diabetes. The aim of this research was to find N‐methylated analogs of the aggregating amylin fragments 18–22, 23–27, and 33–37, which would not themselves be susceptible to aggregation and would inhibit the aggregation of the amyloidogenic cores of the hormone. None of the analogs of fragment 18–22 containing one or two N‐methylated amino acid residues showed any tendency to aggregate. Only the peptide H−F(N−Me)GA(N−Me) IL−OH (6) derived from the 23–27 hIAPP hot spot did not form fibrous structures. All analogs of the 33–37 amylin fragment were characterized by the ability to form aggregates, despite the presence of N‐methylated amino acids in their structures. N‐Methylated peptides 1–5 demonstrated inhibitory properties against the aggregation of fragment 18–22. Aggregation of the amyloidogenic core of 23–27 was significantly inhibited by N‐methylated peptides 1–3 derived from the (18–22) H−HSSNN−OH fragment and by the H−F(N‐Me)GA(N−Me)IL−OH (6) fragment derived from the 23–27 amylin hot spot. Fragment (33–37) H−GSNTY−NH2 was found to be inhibited in the presence of N‐methylated peptides 1–3 derived from the 18–22 fragment and by the double methylated peptide H−F(N−Me)GA(N−Me)IL−OH (6). Research on the possibility of using N‐methylated analogs of amyloidogenic amylin cores as inhibitors of hormone aggregation is ongoing, with a focus on finding the minimum concentration of N‐methylated peptides capable of inhibiting the aggregation of hIAPP hot spots.
中文翻译:

hIAPP 片段 18-22、23-27、33-37 的 N-甲基化类似物抑制激素淀粉样蛋白核心的聚集
胰淀素 (hIAPP) 聚集导致不溶性沉积物的形成,是 II 型糖尿病发展的因素之一。本研究的目的是找到聚集胰淀素片段 18-22、23-27 和 33-37 的 N-甲基化类似物,它们本身不易聚集,并且会抑制激素的淀粉样蛋白核心的聚集. 包含一个或两个 N 甲基化氨基酸残基的片段 18-22 的类似物均未显示任何聚集趋势。只有源自 23-27 hIAPP 热点的肽 H-F(N-Me)GA(N-Me) IL-OH (6) 没有形成纤维结构。尽管结构中存在 N 甲基化氨基酸,但 33-37 胰淀素片段的所有类似物都具有形成聚集体的能力。N-甲基化肽 1-5 显示出对片段 18-22 聚集的抑制特性。来自 (18-22) H-HSSNN-OH 片段的 N-甲基化肽 1-3 和 H-F(N-Me)GA(N-Me) 显着抑制了 23-27 的淀粉样蛋白生成核心的聚集)IL-OH (6) 片段源自 23-27 胰淀素热点。发现片段 (33-37) H-GSNTY-NH2 在存在源自 18-22 片段的 N-甲基化肽 1-3 和双甲基化肽 H-F(N-Me)GA( N-Me)IL-OH (6)。正在研究使用 N-甲基化淀粉样蛋白核心类似物作为激素聚集抑制剂的可能性,重点是找到能够抑制 hIAPP 热点聚集的 N-甲基化肽的最低浓度。来自 (18-22) H-HSSNN-OH 片段的 N-甲基化肽 1-3 和 H-F(N-Me)GA(N-Me) 显着抑制了 23-27 的淀粉样蛋白生成核心的聚集)IL-OH (6) 片段源自 23-27 胰淀素热点。发现片段 (33-37) H-GSNTY-NH2 在存在源自 18-22 片段的 N-甲基化肽 1-3 和双甲基化肽 H-F(N-Me)GA( N-Me)IL-OH (6)。正在研究使用 N-甲基化淀粉样蛋白核心类似物作为激素聚集抑制剂的可能性,重点是找到能够抑制 hIAPP 热点聚集的 N-甲基化肽的最低浓度。来自 (18-22) H-HSSNN-OH 片段的 N-甲基化肽 1-3 和 H-F(N-Me)GA(N-Me) 显着抑制了 23-27 的淀粉样蛋白生成核心的聚集)IL-OH (6) 片段源自 23-27 胰淀素热点。发现片段 (33-37) H-GSNTY-NH2 在存在源自 18-22 片段的 N-甲基化肽 1-3 和双甲基化肽 H-F(N-Me)GA( N-Me)IL-OH (6)。正在研究使用 N-甲基化淀粉样蛋白核心类似物作为激素聚集抑制剂的可能性,重点是找到能够抑制 hIAPP 热点聚集的 N-甲基化肽的最低浓度。
更新日期:2020-12-17
中文翻译:

hIAPP 片段 18-22、23-27、33-37 的 N-甲基化类似物抑制激素淀粉样蛋白核心的聚集
胰淀素 (hIAPP) 聚集导致不溶性沉积物的形成,是 II 型糖尿病发展的因素之一。本研究的目的是找到聚集胰淀素片段 18-22、23-27 和 33-37 的 N-甲基化类似物,它们本身不易聚集,并且会抑制激素的淀粉样蛋白核心的聚集. 包含一个或两个 N 甲基化氨基酸残基的片段 18-22 的类似物均未显示任何聚集趋势。只有源自 23-27 hIAPP 热点的肽 H-F(N-Me)GA(N-Me) IL-OH (6) 没有形成纤维结构。尽管结构中存在 N 甲基化氨基酸,但 33-37 胰淀素片段的所有类似物都具有形成聚集体的能力。N-甲基化肽 1-5 显示出对片段 18-22 聚集的抑制特性。来自 (18-22) H-HSSNN-OH 片段的 N-甲基化肽 1-3 和 H-F(N-Me)GA(N-Me) 显着抑制了 23-27 的淀粉样蛋白生成核心的聚集)IL-OH (6) 片段源自 23-27 胰淀素热点。发现片段 (33-37) H-GSNTY-NH2 在存在源自 18-22 片段的 N-甲基化肽 1-3 和双甲基化肽 H-F(N-Me)GA( N-Me)IL-OH (6)。正在研究使用 N-甲基化淀粉样蛋白核心类似物作为激素聚集抑制剂的可能性,重点是找到能够抑制 hIAPP 热点聚集的 N-甲基化肽的最低浓度。来自 (18-22) H-HSSNN-OH 片段的 N-甲基化肽 1-3 和 H-F(N-Me)GA(N-Me) 显着抑制了 23-27 的淀粉样蛋白生成核心的聚集)IL-OH (6) 片段源自 23-27 胰淀素热点。发现片段 (33-37) H-GSNTY-NH2 在存在源自 18-22 片段的 N-甲基化肽 1-3 和双甲基化肽 H-F(N-Me)GA( N-Me)IL-OH (6)。正在研究使用 N-甲基化淀粉样蛋白核心类似物作为激素聚集抑制剂的可能性,重点是找到能够抑制 hIAPP 热点聚集的 N-甲基化肽的最低浓度。来自 (18-22) H-HSSNN-OH 片段的 N-甲基化肽 1-3 和 H-F(N-Me)GA(N-Me) 显着抑制了 23-27 的淀粉样蛋白生成核心的聚集)IL-OH (6) 片段源自 23-27 胰淀素热点。发现片段 (33-37) H-GSNTY-NH2 在存在源自 18-22 片段的 N-甲基化肽 1-3 和双甲基化肽 H-F(N-Me)GA( N-Me)IL-OH (6)。正在研究使用 N-甲基化淀粉样蛋白核心类似物作为激素聚集抑制剂的可能性,重点是找到能够抑制 hIAPP 热点聚集的 N-甲基化肽的最低浓度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号