当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DFT Mechanistic Study on Palladium‐Catalyzed Redox‐Neutral Hydroarylation of Unactivated Alkenes with Arylboronic Acids
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-12-16 , DOI: 10.1002/ajoc.202000647 Jianguo Zhou 1 , Yongzhu Zhou 1, 2 , Yanxia Li 1 , Jie Zhang 1 , Lei Zhang 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-12-16 , DOI: 10.1002/ajoc.202000647 Jianguo Zhou 1 , Yongzhu Zhou 1, 2 , Yanxia Li 1 , Jie Zhang 1 , Lei Zhang 1
Affiliation
|
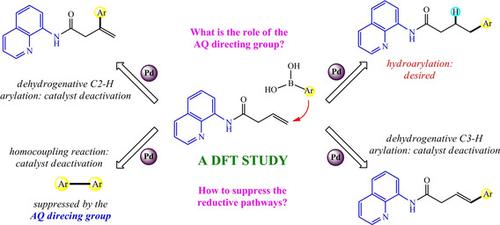
Density functional calculations were carried out on the mechanism of a palladium‐catalyzed hydroarylation reaction of β,γ‐unsaturated carbonyl compounds with arylboronic acids reported by Engle's group. The proposed reaction pathway consists of several key mechanistic steps, including the chelation of substrate 1 a, transmetalation of organoboron 2 a, migratory insertion, protodepalladation, and dechelation of product 3 a, among which the transmetalation step is rate‐determining with a free‐energy barrier of 27.0 kcal/mol. Experimental researchers proposed that the chelation of Daugulis's 8‐aminoquinoline (AQ) directing group facilitated the desired hydroarylation in a γ‐selective manner. However, the present study revealed that the chelation of AQ indeed retarded the delivery of the aryl group to the double bond and the regioselectivity was not necessarily the result of this group. Indeed, it could effectively prevent the homocoupling of 2 a, because the homocoupling was predicted to be easier than the desired hydroarylation in the absence of AQ. Additionally, some reductive coupling pathways between 1 a and 2 a were designed, and the competition of them was discussed in great detail. Lastly, the extension of this synthetic strategy to other types of alkenes was predicted.
中文翻译:

DFT机理研究未活化烯烃与芳基硼酸的钯催化氧化还原-中性加氢芳基化
密度泛函计算是根据恩格尔小组报告的钯催化β,γ-不饱和羰基化合物与芳基硼酸的加氢芳基化反应的机理进行的。所提出的反应途径包括几个关键机理步骤,包括衬底的螯合1,有机硼的转移金属化2,迁移插入,protodepalladation,和dechelation的产品3一个,其中的重金属化步骤是通过27.0 kcal / mol的自由能垒确定速度的。实验研究人员提出,道格利斯8-氨基喹啉(AQ)导向基团的螯合以γ-选择性方式促进了所需的氢芳基化作用。然而,本研究表明,AQ的螯合确实延迟了芳基向双键的传递,区域选择性不一定是该基团的结果。实际上,它可以有效防止2 a的均偶联,因为据预测,在没有AQ的情况下,均偶联比所需的氢芳基化更容易。另外,之间的一些还原偶联通路1和2中的设计,并详细讨论了它们的竞争。最后,预计了该合成策略可扩展至其他类型的烯烃。
更新日期:2021-02-10
中文翻译:

DFT机理研究未活化烯烃与芳基硼酸的钯催化氧化还原-中性加氢芳基化
密度泛函计算是根据恩格尔小组报告的钯催化β,γ-不饱和羰基化合物与芳基硼酸的加氢芳基化反应的机理进行的。所提出的反应途径包括几个关键机理步骤,包括衬底的螯合1,有机硼的转移金属化2,迁移插入,protodepalladation,和dechelation的产品3一个,其中的重金属化步骤是通过27.0 kcal / mol的自由能垒确定速度的。实验研究人员提出,道格利斯8-氨基喹啉(AQ)导向基团的螯合以γ-选择性方式促进了所需的氢芳基化作用。然而,本研究表明,AQ的螯合确实延迟了芳基向双键的传递,区域选择性不一定是该基团的结果。实际上,它可以有效防止2 a的均偶联,因为据预测,在没有AQ的情况下,均偶联比所需的氢芳基化更容易。另外,之间的一些还原偶联通路1和2中的设计,并详细讨论了它们的竞争。最后,预计了该合成策略可扩展至其他类型的烯烃。


























 京公网安备 11010802027423号
京公网安备 11010802027423号