Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-12-17 , DOI: 10.1016/j.tetlet.2020.152717 Paige J Monsen 1 , Frederick A Luzzio 1
|
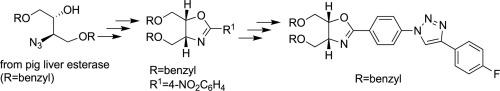
Aryl-substituted esters of a racemic diprotected 2-azido-1-alkanol were submitted to the Staudinger/aza-Wittig reaction in order to assess scope and establish conditions for their cyclization to the corresponding 2,4,5-trisubstituted oxazolines. Following the cyclization study, the (2R,3R)-antipode of the azidoalkanol was obtained in high ee by incubation of the corresponding racemic azidoacetate with pig liver esterase (PLE). The p-nitrobenzoate of the enantioenriched 2-azido-1-alcohol was cyclized by the Staudinger/aza-Wittig to give the corresponding (4R,5R)-disubstituted-2-(4-nitrophenyl) oxazoline. Selective reduction of the nitrophenyloxazoline to the corresponding aminophenyloxazoline using aluminum amalgam followed by direct azidation of the 2-(4-aminophenyl) moiety provided the corresponding (4R,5R)-2-(4-azidophenyl) oxazoline derivative. The azidophenyl oxazoline was reacted with a proven click partner 4-ethynylfluorobenzene under copper/sodium ascorbate mediation to provide the click triazole product in high yield.
中文翻译:

用于点击化学的立体定义的 2-(叠氮基苯基)恶唑啉的化学酶促途径
将外消旋二保护 2-叠氮基-1-烷醇的芳基取代酯进行施陶丁格/氮杂-维蒂希反应,以评估其环化为相应的 2,4,5-三取代恶唑啉的范围并建立条件。在环化研究之后,通过将相应的外消旋叠氮乙酸酯与猪肝酯酶 (PLE) 温育,以高 ee 获得叠氮烷醇的 (2 R, 3 R )-对映体。对映体富集的 2-叠氮基-1-醇的对硝基苯甲酸酯通过 Staudinger/aza-Wittig 环化得到相应的 (4 R ,5 R)-二取代的-2-(4-硝基苯基)恶唑啉。使用铝汞齐将硝基苯基恶唑啉选择性还原为相应的氨基苯基恶唑啉,然后将 2-(4-氨基苯基) 部分直接叠氮化,提供相应的 (4 R ,5 R )-2-(4-叠氮基苯基) 恶唑啉衍生物。在铜/抗坏血酸钠的介导下,叠氮苯基恶唑啉与经过验证的点击伙伴 4-乙炔基氟苯反应,以高产率提供点击三唑产物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号