Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.3 ) Pub Date : 2020-12-17 , DOI: 10.1016/j.jphotochem.2020.113103 Zhengyan Zhao , Xiaolu Zhang , Xuedan Song , Ce Hao
|
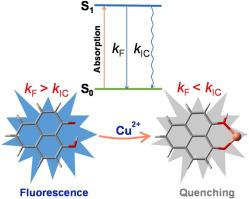
A combination of experimental and computational methods was used to study the Cu2+ ions recognition by 9-hydroxyphenal-1-one carbon quantum dots (HPHN-CQDs). HPHN-CQDs were proved to be an efficient fluorescent probe that can detect Cu2+ ions with high selectivity and sensitivity, and the detection limit was 4.8 nM. We performed a detailed analysis of the fluorescence quenching mechanism of HPHN-CQDs by means of density functional theory (DFT) and time-dependent density functional theory (TDDFT). The orbital interaction diagram indicated that the presence of Cu2+ can affect the luminescence behavior of HPHN by changing its orbital distribution. In addition, the electron was transferred from the excited HPHN to the 3d orbital of Cu2+, which would hinder the recombination of electron-hole pairs and promote the fluorescence quenching of HPHN-CQDs. The photophysical process revealed that the fluorescence rate constant of HPHN-Cu2+ was substantially decreased compared with that of HPHN, which is consistent with the experimental results.
中文翻译:

Cu 2+离子对9-羟基苯-1-酮碳量子点的荧光猝灭机理:实验和计算研究
实验和计算方法的组合用于研究9-羟基苯-1-基碳量子点(HPHN-CQDs)对Cu 2+离子的识别。事实证明,HPHN-CQD是一种高效的荧光探针,可以高选择性和高灵敏度地检测Cu 2+离子,检测限为4.8 nM。我们通过密度泛函理论(DFT)和时变密度泛函理论(TDDFT)对HPHN-CQD的荧光猝灭机理进行了详细分析。轨道相互作用图表明,Cu 2+的存在可以通过改变HPHN的轨道分布来影响其发光行为。此外,电子从激发的HPHN转移到Cu 2+的3d轨道,这将阻碍电子-空穴对的重组并促进HPHN-CQDs的荧光猝灭。光物理过程表明,与HPHN相比,HPHN-Cu 2+的荧光速率常数大大降低,这与实验结果相符。


























 京公网安备 11010802027423号
京公网安备 11010802027423号