当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Binding and Reduced Immunogenicity of Glycoconjugates Prepared via Solid-State Photoactivation of Aliphatic Diazirine Carbohydrates
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-12-16 , DOI: 10.1021/acs.bioconjchem.0c00555 Molly D Congdon 1 , Jeffrey C Gildersleeve 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2020-12-16 , DOI: 10.1021/acs.bioconjchem.0c00555 Molly D Congdon 1 , Jeffrey C Gildersleeve 1
Affiliation
|
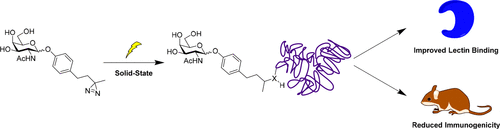
Biological conjugation is an important tool employed for many basic research and clinical applications. While useful, common methods of biological conjugation suffer from a variety of limitations, such as (a) requiring the presence of specific surface-exposed residues, such as lysines or cysteines, (b) reducing protein activity, and/or (c) reducing protein stability and solubility. Use of photoreactive moieties including diazirines, azides, and benzophenones provide an alternative, mild approach to conjugation. Upon irradiation with UV and visible light, these functionalities generate highly reactive carbenes, nitrenes, and radical intermediates. Many of these will couple to proteins in a non-amino-acid-specific manner. The main hurdle for photoactivated biological conjugation is very low yield. In this study, we developed a solid-state method to increase conjugation efficiency of diazirine-containing carbohydrates to proteins. Using this methodology, we produced multivalent carbohydrate–protein conjugates with unaltered protein charge and secondary structure. Compared to carbohydrate conjugates prepared with amide linkages to lysine residues using standard NHS conjugation, the photoreactive prepared conjugates displayed up to 100-fold improved binding to lectins and diminished immunogenicity in mice. These results indicate that photoreactive bioconjugation could be especially useful for in vivo applications, such as lectin targeting, where high binding affinity and low immunogenicity are desired.
中文翻译:

通过脂肪族二氮丙啶碳水化合物的固态光活化制备的糖缀合物的结合增强和免疫原性降低
生物偶联是用于许多基础研究和临床应用的重要工具。虽然有用,但常见的生物偶联方法受到各种限制,例如 (a) 需要存在特定的表面暴露残基,例如赖氨酸或半胱氨酸,(b) 降低蛋白质活性,和/或 (c) 减少蛋白质稳定性和溶解度。使用包括二氮丙啶、叠氮化物和二苯甲酮在内的光反应性部分提供了另一种温和的缀合方法。在紫外线和可见光照射下,这些官能团会产生高反应性卡宾、氮烯和自由基中间体。其中许多将以非氨基酸特异性方式与蛋白质偶联。光活化生物偶联的主要障碍是非常低的产率。在这项研究中,我们开发了一种固态方法来提高含二氮丙啶的碳水化合物与蛋白质的结合效率。使用这种方法,我们生产了具有未改变的蛋白质电荷和二级结构的多价碳水化合物-蛋白质缀合物。与使用标准 NHS 缀合物通过酰胺键与赖氨酸残基制备的碳水化合物缀合物相比,制备的光反应性缀合物与凝集素的结合显示出高达 100 倍的改善,并降低了小鼠的免疫原性。这些结果表明,光反应性生物偶联可能特别适用于 与使用标准 NHS 缀合物通过酰胺键与赖氨酸残基制备的碳水化合物缀合物相比,制备的光反应性缀合物与凝集素的结合显示出高达 100 倍的改善,并降低了小鼠的免疫原性。这些结果表明,光反应性生物偶联可能特别适用于 与使用标准 NHS 缀合物通过酰胺键与赖氨酸残基制备的碳水化合物缀合物相比,制备的光反应性缀合物与凝集素的结合显示出高达 100 倍的改善,并降低了小鼠的免疫原性。这些结果表明,光反应性生物偶联可能特别适用于体内应用,例如凝集素靶向,需要高结合亲和力和低免疫原性。
更新日期:2021-01-20
中文翻译:

通过脂肪族二氮丙啶碳水化合物的固态光活化制备的糖缀合物的结合增强和免疫原性降低
生物偶联是用于许多基础研究和临床应用的重要工具。虽然有用,但常见的生物偶联方法受到各种限制,例如 (a) 需要存在特定的表面暴露残基,例如赖氨酸或半胱氨酸,(b) 降低蛋白质活性,和/或 (c) 减少蛋白质稳定性和溶解度。使用包括二氮丙啶、叠氮化物和二苯甲酮在内的光反应性部分提供了另一种温和的缀合方法。在紫外线和可见光照射下,这些官能团会产生高反应性卡宾、氮烯和自由基中间体。其中许多将以非氨基酸特异性方式与蛋白质偶联。光活化生物偶联的主要障碍是非常低的产率。在这项研究中,我们开发了一种固态方法来提高含二氮丙啶的碳水化合物与蛋白质的结合效率。使用这种方法,我们生产了具有未改变的蛋白质电荷和二级结构的多价碳水化合物-蛋白质缀合物。与使用标准 NHS 缀合物通过酰胺键与赖氨酸残基制备的碳水化合物缀合物相比,制备的光反应性缀合物与凝集素的结合显示出高达 100 倍的改善,并降低了小鼠的免疫原性。这些结果表明,光反应性生物偶联可能特别适用于 与使用标准 NHS 缀合物通过酰胺键与赖氨酸残基制备的碳水化合物缀合物相比,制备的光反应性缀合物与凝集素的结合显示出高达 100 倍的改善,并降低了小鼠的免疫原性。这些结果表明,光反应性生物偶联可能特别适用于 与使用标准 NHS 缀合物通过酰胺键与赖氨酸残基制备的碳水化合物缀合物相比,制备的光反应性缀合物与凝集素的结合显示出高达 100 倍的改善,并降低了小鼠的免疫原性。这些结果表明,光反应性生物偶联可能特别适用于体内应用,例如凝集素靶向,需要高结合亲和力和低免疫原性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号