当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Developability Assessment of an Isolated CH2 Immunoglobulin Domain
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-12-16 , DOI: 10.1021/acs.analchem.0c02663 Belinda Pastrana 1 , Sherly Nieves 1 , Wei Li 2, 3 , Xianglei Liu 3 , Dimiter S. Dimitrov 2, 3
Analytical Chemistry ( IF 7.4 ) Pub Date : 2020-12-16 , DOI: 10.1021/acs.analchem.0c02663 Belinda Pastrana 1 , Sherly Nieves 1 , Wei Li 2, 3 , Xianglei Liu 3 , Dimiter S. Dimitrov 2, 3
Affiliation
|
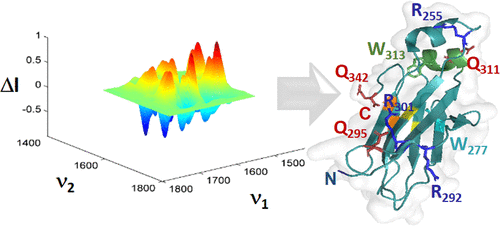
The IgG CH2 domain continues to hold promise for the development of new therapeutic entities because of its bifunctional role as a biomarker and effector protein. The need for further understanding of molecular stability and aggregation in therapeutic proteins has led to the development of a breakthrough quantum cascade laser microscope to allow for real-time comparability assessment of an array of related proteins in solution upon thermal perturbation. Our objective was to perform a comprehensive developability assessment of three similar monoclonal antibody (mAb) fragments: CH2, CH2s, and m01s. The CH2 construct consists of residues Pro238 to Lys340 of the IgG1 heavy chain sequence. CH2s has a 7-residue deletion at the N-terminus and a 16-residue C-terminal extension containing a histidine tag. The m01s construct is identical to CH2s, except for two cysteines introduced at positions 242 and 334. A series of hyperspectral images was acquired during thermal perturbation from 28 to 60 °C for all three proteins in an array. Co-distribution and two-dimensional infrared correlation spectroscopies yielded the mechanism of aggregation and stability for these three proteins. The level of detail is unprecedented, identifying the regions within CH2 and CH2s that are prone to self-association and establishing the differences in stability. Furthermore, CH2 helical segments, β-sheets, β-turns, and random coil regions were less stable than in CH2s and m01s because of the presence of the N-terminal 310-helix and β-turn type III. The engineered disulfide bridge in m01s eliminated the self-association process and rendered this mAb fragment the most stable.
中文翻译:

分离的C H 2免疫球蛋白结构域的可开发性评估
IgG C H 2结构域由于其作为生物标志物和效应蛋白的双重功能而继续为开发新的治疗实体提供前景。对治疗性蛋白质分子稳定性和聚集性的进一步了解的需求导致了突破性的量子级联激光显微镜的发展,以允许在热扰动下实时评估溶液中一系列相关蛋白质的可比性。我们的目标是对三种相似的单克隆抗体(mAb)片段进行全面的可开发性评估:C H 2,C H 2s和m01s。C H 2构建体由IgG1重链序列的残基Pro238至Lys340组成。ç ^ h2s在N-末端具有7个残基的缺失,并且含有一个组氨酸标签的16个残基的C-末端延伸。m01s的构建体与C H 2s相同,不同之处在于在位置242和334引入了两个半胱氨酸。在28°C至60°C的热扰动期间,阵列中的所有三种蛋白质均获得了一系列高光谱图像。共分布和二维红外相关光谱学得出了这三种蛋白质的聚集和稳定性机制。详细程度是空前的,它确定了C H 2和C H 2s中易于自缔合的区域并确定了稳定性的差异。此外,C H由于存在N末端3 10螺旋和III型β匝,因此2个螺旋段,β折叠,β匝和随机线圈区域的稳定性低于C H 2s和m01s 。在m01s中设计的二硫键消除了自缔合过程,并使该mAb片段最稳定。
更新日期:2021-01-26
中文翻译:

分离的C H 2免疫球蛋白结构域的可开发性评估
IgG C H 2结构域由于其作为生物标志物和效应蛋白的双重功能而继续为开发新的治疗实体提供前景。对治疗性蛋白质分子稳定性和聚集性的进一步了解的需求导致了突破性的量子级联激光显微镜的发展,以允许在热扰动下实时评估溶液中一系列相关蛋白质的可比性。我们的目标是对三种相似的单克隆抗体(mAb)片段进行全面的可开发性评估:C H 2,C H 2s和m01s。C H 2构建体由IgG1重链序列的残基Pro238至Lys340组成。ç ^ h2s在N-末端具有7个残基的缺失,并且含有一个组氨酸标签的16个残基的C-末端延伸。m01s的构建体与C H 2s相同,不同之处在于在位置242和334引入了两个半胱氨酸。在28°C至60°C的热扰动期间,阵列中的所有三种蛋白质均获得了一系列高光谱图像。共分布和二维红外相关光谱学得出了这三种蛋白质的聚集和稳定性机制。详细程度是空前的,它确定了C H 2和C H 2s中易于自缔合的区域并确定了稳定性的差异。此外,C H由于存在N末端3 10螺旋和III型β匝,因此2个螺旋段,β折叠,β匝和随机线圈区域的稳定性低于C H 2s和m01s 。在m01s中设计的二硫键消除了自缔合过程,并使该mAb片段最稳定。


























 京公网安备 11010802027423号
京公网安备 11010802027423号