当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adaptive Lipid Immiscibility and Membrane Remodeling Are Active Functional Determinants of Primary Ciliogenesis
Small Methods ( IF 12.4 ) Pub Date : 2020-12-16 , DOI: 10.1002/smtd.202000711 Miguel Bernabé-Rubio 1, 2 , Minerva Bosch-Fortea 1, 3 , Esther García 4, 5 , Jorge Bernardino de la Serna 4, 6, 7 , Miguel A Alonso 1
Small Methods ( IF 12.4 ) Pub Date : 2020-12-16 , DOI: 10.1002/smtd.202000711 Miguel Bernabé-Rubio 1, 2 , Minerva Bosch-Fortea 1, 3 , Esther García 4, 5 , Jorge Bernardino de la Serna 4, 6, 7 , Miguel A Alonso 1
Affiliation
|
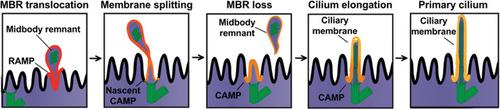
Lipid liquid–liquid immiscibility and its consequent lateral heterogeneity have been observed under thermodynamic equilibrium in model and native membranes. However, cholesterol‐rich membrane domains, sometimes referred to as lipid rafts, are difficult to observe spatiotemporally in live cells. Despite their importance in many biological processes, robust evidence for their existence remains elusive. This is mainly due to the difficulty in simultaneously determining their chemical composition and physicochemical nature, whilst spatiotemporally resolving their nanodomain lifetime and molecular dynamics. In this study, a bespoke method based on super‐resolution stimulated emission depletion (STED) microscopy and raster imaging correlation spectroscopy (RICS) is used to overcome this issue. This methodology, laser interleaved confocal RICS and STED‐RICS (LICSR), enables simultaneous tracking of lipid lateral packing and dynamics at the nanoscale. Previous work indicated that, in polarized epithelial cells, the midbody remnant licenses primary cilium formation through an unidentified mechanism. LICSR shows that lipid immiscibility and its adaptive collective nanoscale self‐assembly are crucial for the midbody remnant to supply condensed membranes to the centrosome for the biogenesis of the ciliary membrane. Hence, this work poses a breakthrough in the field of lipid biology by providing compelling evidence of a functional role for liquid ordered‐like membranes in primary ciliogenesis.
中文翻译:

适应性脂质不混溶性和膜重塑是初级纤毛发生的主动功能决定因素
在模型和天然膜的热力学平衡下观察到脂质液-液不混溶性及其随之而来的横向异质性。然而,富含胆固醇的膜结构域,有时被称为脂筏,很难在活细胞中时空观察。尽管它们在许多生物过程中都很重要,但它们存在的有力证据仍然难以捉摸。这主要是由于难以同时确定它们的化学成分和物理化学性质,同时在时空上解决它们的纳米域寿命和分子动力学。在这项研究中,一种基于超分辨率受激发射损耗 (STED) 显微镜和光栅成像相关光谱 (RICS) 的定制方法被用来克服这个问题。这种方法论,激光交错共聚焦 RICS 和 STED-RICS (LICSR),能够同时跟踪纳米级的脂质横向堆积和动力学。以前的工作表明,在极化的上皮细胞中,中间体残余物通过一种未知的机制允许初级纤毛形成。LICSR 表明,脂质不混溶性及其适应性集体纳米级自组装对于中间体残余物向中心体提供凝聚膜以促进睫状膜的生物发生至关重要。因此,这项工作通过提供令人信服的证据证明液体有序样膜在初级纤毛发生中的功能作用,在脂质生物学领域取得了突破。中间体残余通过一种未知的机制许可初级纤毛形成。LICSR 表明,脂质不混溶性及其适应性集体纳米级自组装对于中间体残余物向中心体提供凝聚膜以促进睫状膜的生物发生至关重要。因此,这项工作通过提供令人信服的证据证明液体有序样膜在初级纤毛发生中的功能作用,在脂质生物学领域取得了突破。中间体残余通过一种未知的机制许可初级纤毛形成。LICSR 表明,脂质不混溶性及其适应性集体纳米级自组装对于中间体残余物向中心体提供凝聚膜以促进睫状膜的生物发生至关重要。因此,这项工作通过提供令人信服的证据证明液体有序样膜在初级纤毛发生中的功能作用,在脂质生物学领域取得了突破。
更新日期:2021-02-12
中文翻译:

适应性脂质不混溶性和膜重塑是初级纤毛发生的主动功能决定因素
在模型和天然膜的热力学平衡下观察到脂质液-液不混溶性及其随之而来的横向异质性。然而,富含胆固醇的膜结构域,有时被称为脂筏,很难在活细胞中时空观察。尽管它们在许多生物过程中都很重要,但它们存在的有力证据仍然难以捉摸。这主要是由于难以同时确定它们的化学成分和物理化学性质,同时在时空上解决它们的纳米域寿命和分子动力学。在这项研究中,一种基于超分辨率受激发射损耗 (STED) 显微镜和光栅成像相关光谱 (RICS) 的定制方法被用来克服这个问题。这种方法论,激光交错共聚焦 RICS 和 STED-RICS (LICSR),能够同时跟踪纳米级的脂质横向堆积和动力学。以前的工作表明,在极化的上皮细胞中,中间体残余物通过一种未知的机制允许初级纤毛形成。LICSR 表明,脂质不混溶性及其适应性集体纳米级自组装对于中间体残余物向中心体提供凝聚膜以促进睫状膜的生物发生至关重要。因此,这项工作通过提供令人信服的证据证明液体有序样膜在初级纤毛发生中的功能作用,在脂质生物学领域取得了突破。中间体残余通过一种未知的机制许可初级纤毛形成。LICSR 表明,脂质不混溶性及其适应性集体纳米级自组装对于中间体残余物向中心体提供凝聚膜以促进睫状膜的生物发生至关重要。因此,这项工作通过提供令人信服的证据证明液体有序样膜在初级纤毛发生中的功能作用,在脂质生物学领域取得了突破。中间体残余通过一种未知的机制许可初级纤毛形成。LICSR 表明,脂质不混溶性及其适应性集体纳米级自组装对于中间体残余物向中心体提供凝聚膜以促进睫状膜的生物发生至关重要。因此,这项工作通过提供令人信服的证据证明液体有序样膜在初级纤毛发生中的功能作用,在脂质生物学领域取得了突破。


























 京公网安备 11010802027423号
京公网安备 11010802027423号