Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2020-12-16 , DOI: 10.1016/j.xcrp.2020.100274 Kjell Jorner , Wangchuk Rabten , Tomas Slanina , Nathalie Proos Vedin , Sara Sillén , Jufang Wu Ludvigsson , Henrik Ottosson , Per-Ola Norrby
|
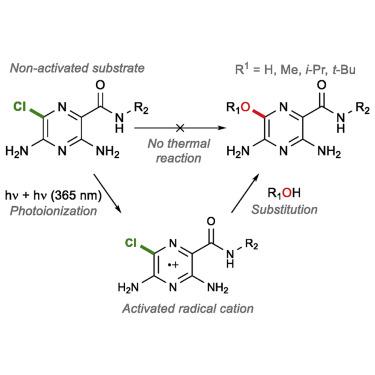
Haloaromatic drug molecules of the amiloride family are plagued by photodegradation with associated toxicity. Herein, we report on the photodegradation of analogs of amiloride, which are known to undergo photosubstitution in water. Model compounds built on the same scaffold undergo clean photosubstitution also in alcoholic solvent, where a certain amount of photodehalogenation is normally expected. Available evidence points to a mechanism starting with photoexcitation followed by photoionization to give a radical cation intermediate. Subsequent substitution reaction with the protic solvent is assisted by a general base, possibly strengthened by the proximal solvated electron. Recombination with the solvated electron generates the observed product. Quantum chemical computations reveal that excited state antiaromaticity is relieved when an electron is ejected from the photoexcited molecule by the second photon. The mechanism indicated here could have wide applicability to photoinduced degradation of similar heteroaromatic compounds in the environment, as well as to a class of increasingly popular synthetic photoredox methods.
中文翻译:

通过阿米洛利衍生物的顺序光子吸收和光电离降解药物
阿米洛利家族的卤代芳香族药物分子受到光降解以及相关毒性的困扰。在此,我们报道了阿米洛利类似物的光降解作用,已知该阿米洛利在水中会经历光解。在相同的支架上构建的模型化合物也会在酒精溶剂中经历干净的光解,其中通常预期会发生一定程度的光脱卤。现有的证据指出了一种机制,该机制从光激发开始,然后进行光离子化,从而产生自由基阳离子中间体。随后与质子溶剂的取代反应由一般的碱辅助,可能由近端的溶剂化电子增强。用溶剂化电子进行重组产生了观察到的产物。量子化学计算表明,当电子被第二光子从光激发分子中射出时,激发态抗芳香性得到缓解。此处指出的机理可能对环境中类似杂芳族化合物的光诱导降解以及一类越来越流行的合成光氧化还原方法具有广泛的适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号