Nitric Oxide ( IF 3.9 ) Pub Date : 2020-12-15 , DOI: 10.1016/j.niox.2020.12.002 Ettore Lignelli 1 , Francesco Palumbo 1 , Selahattin Görkem Bayindir 1 , Noriyuki Nagahara 2 , István Vadász 3 , Susanne Herold 3 , Werner Seeger 4 , Rory E Morty 5
|
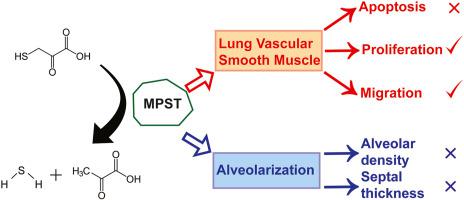
Along with nitric oxide (NO), the gasotransmitters carbon monoxide (CO) and hydrogen sulfide (H2S) are emerging as potentially important players in newborn physiology, as mediators of newborn disease, and as new therapeutic modalities. Several recent studies have addressed H2S in particular in animal models of bronchopulmonary dysplasia (BPD), a common complication of preterm birth where oxygen toxicity stunts lung development. In those studies, exogenous H2S attenuated the impact of oxygen toxicity on lung development, and two H2S-generating enzymes were documented to affect pulmonary vascular development. H2S is directly generated endogenously by three enzymes, one of which, 3-mercaptopyruvate sulfurtransferase (MPST), has not been studied in the lung. In a hyperoxia-based animal model of BPD, oxygen exposure deregulated MPST expression during post-natal lung development, where MPST was localized to the smooth muscle layer of the pulmonary vessels in developing lungs. siRNA-mediated abrogation of MPST expression in human pulmonary artery smooth muscle cells in vitro limited baseline cell migration and cell proliferation, without affecting apoptosis or cell viability. In vivo, MPST was dispensable for normal lung development in Mpst−/−mice, and MPST did not contribute to stunted lung development driven by hyperoxia exposure, assessed by design-based stereology. These data demonstrate novel roles for MPST in pulmonary vascular smooth muscle cell physiology. The potential caveats of using Mpst−/- mice to study normal and aberrant lung development are also discussed, highlighting the possible confounding, compensatory effects of other H2S-generating enzymes that are present alongside MPST in the smooth muscle compartment of developing pulmonary vessels.
中文翻译:

产生H 2 S的酶3-巯基丙酮酸硫转移酶调节肺血管平滑肌细胞的迁移和增殖,但不影响正常或异常的肺发育
一氧化碳(CO)和硫化氢(H 2 S)与一氧化氮(NO)一起,已成为新生儿生理学中潜在的重要角色,新生儿疾病的介质和新的治疗方式。最近的几项研究已针对H 2 S进行了研究,特别是在支气管肺发育不良(BPD)的动物模型中,这是早产的常见并发症,其中氧中毒阻碍了肺的发育。在那些研究中,外源性H 2 S减弱了氧气毒性对肺部发育的影响,并且有两种H 2 S生成酶被证明会影响肺血管的发育。高2S是由三种酶直接内源性产生的,其中一种在肺中尚未研究过3-巯基丙酮酸硫转移酶(MPST)。在以高氧为基础的BPD动物模型中,氧暴露会降低出生后肺部发育过程中MPST的表达,其中MPST局限于发育中肺部肺血管的平滑肌层。在体外, siRNA介导的人肺动脉平滑肌细胞中MPST表达的消除限制了基线细胞迁移和细胞增殖,而不影响细胞凋亡或细胞生存力。在体内,MPST对于Mpst -/-的正常肺发育是必不可少的小鼠和MPST并没有导致高氧暴露所致的发育迟缓的肺发育。这些数据证明了MPST在肺血管平滑肌细胞生理中的新作用。还讨论了使用Mpst -/-小鼠研究正常和异常肺发育的潜在警告,并强调了与MPST并存的其他H 2 S生成酶在发育中的肺血管平滑肌腔中可能产生混杂,补偿作用。。


























 京公网安备 11010802027423号
京公网安备 11010802027423号