Organic Materials Pub Date : 2020-12-14 , DOI: 10.1055/s-0040-1721102 Anjan Bedi 1 , Linda J. W. Shimon 2 , Benny Bogoslavsky 1 , Ori Gidron 1
|
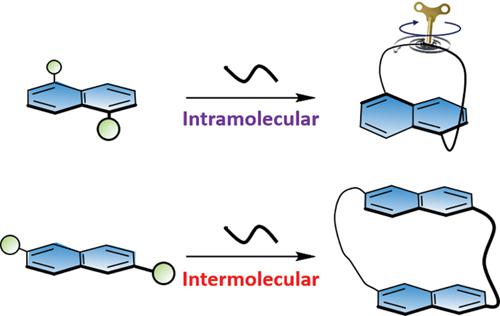
Twisting anthracene and higher acenes can alter their optical, magnetic, and electronic properties. To test the effect of twisting on the lower homologue, naphthalene, we synthesized tethered naphthalenophanes bearing alkyl bridges. Both X-ray structure and DFT calculations show that hexyl and butyl bridges induce a 6° and 12° end-to-end twist on the naphthalene unit, respectively. Attempts to increase the twisting further using shorter tethers resulted in an elimination product. Enantiomerically pure naphthalenophanes display strong chiroptical properties, which intensify with increasing twist. Attempts to induce bending, rather than twisting, using the same synthetic methodology, resulted in intermolecular dimerization, yielding macrocyclic naphthalenes. This work highlights the importance of steric hindrance in the synthesis of curved cyclophanes using the bridge formation approach.
中文翻译:

扭曲比弯曲更容易:萘基萘合成的桥形成方法的范围
扭曲蒽和高级并六苯可以改变其光学,磁性和电子性质。为了测试扭曲对较低同系物萘的影响,我们合成了带有烷基桥的拴系萘甲酸酯。X射线结构和DFT计算均表明,己基和丁基桥分别在萘单元上引起6°和12°的端到端扭曲。尝试使用更短的系绳进一步增加扭曲度会产生消除产物。对映体纯的萘甲酚显示出很强的手性,随着扭曲的增加而增强。尝试使用相同的合成方法诱导弯曲而不是扭曲,导致分子间二聚化,生成大环萘。


























 京公网安备 11010802027423号
京公网安备 11010802027423号