当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase I clinical trial of intra‐bone marrow cotransplantation of mesenchymal stem cells in cord blood transplantation
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-12-14 , DOI: 10.1002/sctm.20-0381 Tatsunori Goto 1 , Makoto Murata 1 , Tetsuya Nishida 1 , Seitaro Terakura 1 , Sonoko Kamoshita 1 , Yuichi Ishikawa 1 , Yoko Ushijima 1 , Yoshiya Adachi 1 , Satoshi Suzuki 2 , Katsuyoshi Kato 2 , Akihiro Hirakawa 2 , Satoshi Nishiwaki 2 , Nobuhiro Nishio 2 , Yoshiyuki Takahashi 3 , Yoshihisa Kodera 4 , Tadashi Matsushita 5 , Hitoshi Kiyoi 1
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-12-14 , DOI: 10.1002/sctm.20-0381 Tatsunori Goto 1 , Makoto Murata 1 , Tetsuya Nishida 1 , Seitaro Terakura 1 , Sonoko Kamoshita 1 , Yuichi Ishikawa 1 , Yoko Ushijima 1 , Yoshiya Adachi 1 , Satoshi Suzuki 2 , Katsuyoshi Kato 2 , Akihiro Hirakawa 2 , Satoshi Nishiwaki 2 , Nobuhiro Nishio 2 , Yoshiyuki Takahashi 3 , Yoshihisa Kodera 4 , Tadashi Matsushita 5 , Hitoshi Kiyoi 1
Affiliation
|
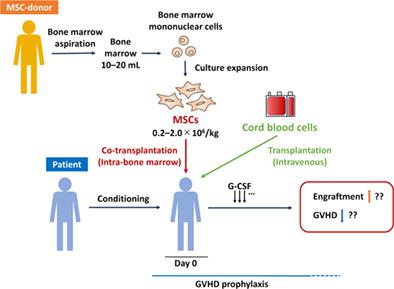
Mesenchymal stem cells (MSCs) have immunomodulatory properties and support hematopoiesis in the bone marrow (BM). To develop a new strategy to not only prevent graft‐vs‐host disease (GVHD) but also to enhance engraftment, a phase I trial of cord blood transplantation (CBT) combined with intra‐BM injection of MSCs (MSC‐CBT) was designed. Third‐party BM‐derived MSCs were injected intra‐BM on the day of CBT. The conditioning regimen varied according to patient characteristics. GVHD prophylaxis was tacrolimus and methotrexate. The primary endpoint was toxicity related to intra‐BM injection of MSCs. Clinical outcomes were compared with those of six controls who received CBT alone. Five adult patients received MSC‐CBT, and no adverse events related to intra‐BM injection of MSCs were observed. All patients achieved neutrophil, reticulocyte, and platelet recoveries, with median times to recoveries of 21, 35, and 38 days, respectively, comparable with controls. Grade II‐IV acute GVHD developed in three controls but not in MSC‐CBT patients. No patients developed chronic GVHD in both groups. At 1 year after transplantation, all MSC‐CBT patients survived without relapse. This study shows the safety of MSC‐CBT, and the findings also suggest that cotransplantation of MSCs may prevent GVHD with no inhibition of engraftment. This trial was registered at the University Hospital Medical Information Network Clinical Trials Registry as number 000024291.
中文翻译:

间充质干细胞在脐血移植中的骨髓内共移植I期临床试验
间充质干细胞 (MSCs) 具有免疫调节特性并支持骨髓 (BM) 中的造血功能。为了开发一种新的策略,不仅可以预防移植物抗宿主病 (GVHD),而且可以增强移植,设计了脐带血移植 (CBT) 与骨髓内注射 MSC (MSC-CBT) 相结合的 I 期试验. 在 CBT 当天将第三方 BM 衍生的 MSCs 注射到 BM 内。预处理方案根据患者特征而变化。GVHD 预防是他克莫司和甲氨蝶呤。主要终点是与骨髓内注射 MSCs 相关的毒性。临床结果与仅接受 CBT 的六名对照进行比较。5 名成年患者接受了 MSC-CBT,未观察到与 MSCs 内注射相关的不良事件。所有患者均达到中性粒细胞、网织红细胞、和血小板恢复,中位恢复时间分别为 21、35 和 38 天,与对照组相当。II-IV 级急性 GVHD 在三个对照组中发生,但在 MSC-CBT 患者中没有。两组患者均未出现慢性 GVHD。移植后 1 年,所有 MSC-CBT 患者均存活且未复发。这项研究显示了 MSC-CBT 的安全性,研究结果还表明,MSCs 的共同移植可以预防 GVHD,而不会抑制植入。该试验已在大学医院医学信息网络临床试验注册中心注册,编号为 000024291。移植后 1 年,所有 MSC-CBT 患者均存活且未复发。这项研究显示了 MSC-CBT 的安全性,研究结果还表明,MSCs 的共同移植可以预防 GVHD,而不会抑制植入。该试验已在大学医院医学信息网络临床试验注册中心注册,编号为 000024291。移植后 1 年,所有 MSC-CBT 患者均存活且未复发。这项研究显示了 MSC-CBT 的安全性,研究结果还表明,MSCs 的共同移植可以预防 GVHD,而不会抑制植入。该试验已在大学医院医学信息网络临床试验注册中心注册,编号为 000024291。
更新日期:2020-12-14
中文翻译:

间充质干细胞在脐血移植中的骨髓内共移植I期临床试验
间充质干细胞 (MSCs) 具有免疫调节特性并支持骨髓 (BM) 中的造血功能。为了开发一种新的策略,不仅可以预防移植物抗宿主病 (GVHD),而且可以增强移植,设计了脐带血移植 (CBT) 与骨髓内注射 MSC (MSC-CBT) 相结合的 I 期试验. 在 CBT 当天将第三方 BM 衍生的 MSCs 注射到 BM 内。预处理方案根据患者特征而变化。GVHD 预防是他克莫司和甲氨蝶呤。主要终点是与骨髓内注射 MSCs 相关的毒性。临床结果与仅接受 CBT 的六名对照进行比较。5 名成年患者接受了 MSC-CBT,未观察到与 MSCs 内注射相关的不良事件。所有患者均达到中性粒细胞、网织红细胞、和血小板恢复,中位恢复时间分别为 21、35 和 38 天,与对照组相当。II-IV 级急性 GVHD 在三个对照组中发生,但在 MSC-CBT 患者中没有。两组患者均未出现慢性 GVHD。移植后 1 年,所有 MSC-CBT 患者均存活且未复发。这项研究显示了 MSC-CBT 的安全性,研究结果还表明,MSCs 的共同移植可以预防 GVHD,而不会抑制植入。该试验已在大学医院医学信息网络临床试验注册中心注册,编号为 000024291。移植后 1 年,所有 MSC-CBT 患者均存活且未复发。这项研究显示了 MSC-CBT 的安全性,研究结果还表明,MSCs 的共同移植可以预防 GVHD,而不会抑制植入。该试验已在大学医院医学信息网络临床试验注册中心注册,编号为 000024291。移植后 1 年,所有 MSC-CBT 患者均存活且未复发。这项研究显示了 MSC-CBT 的安全性,研究结果还表明,MSCs 的共同移植可以预防 GVHD,而不会抑制植入。该试验已在大学医院医学信息网络临床试验注册中心注册,编号为 000024291。


























 京公网安备 11010802027423号
京公网安备 11010802027423号