当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reusable Silica‐Supported Ammonium BINSate Catalysts for Enantio‐ and Diastereoselective Friedel–Crafts‐Type Double Aminoalkylation of N‐Alkylpyrroles with Aldimines
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-12-13 , DOI: 10.1002/ajoc.202000603 Manabu Hatano 1 , Xue Zhao 2 , Takuya Mochizuki 2 , Kyogo Maeda 3 , Ken Motokura 3 , Kazuaki Ishihara 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-12-13 , DOI: 10.1002/ajoc.202000603 Manabu Hatano 1 , Xue Zhao 2 , Takuya Mochizuki 2 , Kyogo Maeda 3 , Ken Motokura 3 , Kazuaki Ishihara 2
Affiliation
|
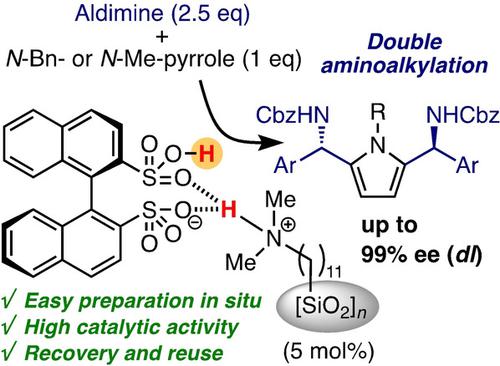
Silica‐supported ammonium (R)‐BINSate catalysts for the enantio‐ and diastereoselective Friedel–Crafts‐type double aminoalkylation of N‐benzyl‐ or N‐methylpyrrole with aldimines were developed. The present heterogeneous catalysts showed high catalytic activity compared to our previous homogeneous (R)‐BINSA ammonium catalysts, which were effective for single aminoalkylation. Simple aldimines could be used, and the corresponding pyrrole‐derived chiral C2‐symmetric triamines were obtained in the dl‐form with good to extremely high enantioselectivities. The heterogeneous catalyst could be easily recovered and reused three times without any loss of catalytic activity or enantiocontrol. An XPS analysis supported precise preparation of the catalysts in situ and with good quality after recycling three times. From the perspective of modern green chemistry with fine asymmetric organocatalysis, the development of such chiral strong Brønsted acid catalysts might be useful for both laboratory and industrial applications.
中文翻译:

可重复使用的二氧化硅支持的双磺酸铵催化剂,用于N-烷基吡咯与醛亚胺的对映和非对映选择性Friedel-Crafts型双氨基烷基化反应
开发了用于N-苄基或N-甲基吡咯与醛亚胺的对映和非对映选择性Friedel-Crafts型双氨基烷基化反应的二氧化硅支撑的铵盐(R)-BINSate催化剂。与我们以前的均相(R)-BINSA铵催化剂相比,本发明的多相催化剂显示出高催化活性,后者对单个氨基烷基化有效。可以使用简单的亚胺,并在dl中获得相应的吡咯衍生的手性C 2对称三胺。具有良好至极高对映选择性的形式。该非均相催化剂可以容易地回收并重复使用三次,而不会损失任何催化活性或对映体。XPS分析支持精确地原位制备催化剂, 并经过3次循环后仍具有良好的质量。从具有精细不对称有机催化作用的现代绿色化学的角度来看,这种手性强布朗斯台德酸催化剂的开发可能对实验室和工业应用都是有用的。
更新日期:2021-02-10
中文翻译:

可重复使用的二氧化硅支持的双磺酸铵催化剂,用于N-烷基吡咯与醛亚胺的对映和非对映选择性Friedel-Crafts型双氨基烷基化反应
开发了用于N-苄基或N-甲基吡咯与醛亚胺的对映和非对映选择性Friedel-Crafts型双氨基烷基化反应的二氧化硅支撑的铵盐(R)-BINSate催化剂。与我们以前的均相(R)-BINSA铵催化剂相比,本发明的多相催化剂显示出高催化活性,后者对单个氨基烷基化有效。可以使用简单的亚胺,并在dl中获得相应的吡咯衍生的手性C 2对称三胺。具有良好至极高对映选择性的形式。该非均相催化剂可以容易地回收并重复使用三次,而不会损失任何催化活性或对映体。XPS分析支持精确地原位制备催化剂, 并经过3次循环后仍具有良好的质量。从具有精细不对称有机催化作用的现代绿色化学的角度来看,这种手性强布朗斯台德酸催化剂的开发可能对实验室和工业应用都是有用的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号