当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of reductant type on the combustion synthesis of NiB
Solid State Sciences ( IF 3.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.solidstatesciences.2020.106447 Ömür Can Odabaş , Mehmet Buğdaycı , Selçuk Kan , Ahmet Turan , Onuralp Yücel
Solid State Sciences ( IF 3.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.solidstatesciences.2020.106447 Ömür Can Odabaş , Mehmet Buğdaycı , Selçuk Kan , Ahmet Turan , Onuralp Yücel
|
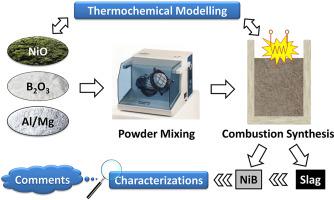
Abstract Ni-B intermetallic alloys are important filler materials which are used in brazing applications. Carbothermic and aluminothermic synthesis routes are main methods to produce NiB. Carbothermic synthesis is carried out in electric arc furnaces and consumes high amount of energy whereas aluminothermic synthesis mainly uses its own reaction energy. Thus, via aluminothermic synthesis, the consumption of energy drops. Aluminothermic synthesis is a sub-group of combustion synthesis methods and, its name is based on the Al which is used as reductant in reactions. In the present study, aluminothermic synthesis conditions of NiB (mainly containing 15% B by wt.) were investigated for increasing B2O3 stoichiometry in reactants. Moreover, some experiments were conducted to understand the magnesiothermic synthesis conditions, as an alternative reductant. Results were compared to each other and to thermochemical simulations which were carried out by using HSC Chemistry 6.12 and FactSage 7.1 softwares. Chemical analysis, SEM-EDS and XRD were the main techniques to characterize raw materials and the products. Increasing B2O3 stoichiometry and the use of the Mg as reductant positively affected the chemical content of obtained NiB phases although metal recovery yields were lower for magnesiothermic experiments than that of aluminothermic experiments. For instance, in the magnesiothermic experiment carried out with 100% B2O3 stoichiometry, the obtained metallic fraction consisted of 84.2% Ni, 15.1% B and 0.1% Mg.
中文翻译:

还原剂类型对NiB燃烧合成的影响
摘要 Ni-B金属间合金是重要的钎焊材料。碳热和铝热合成路线是生产 NiB 的主要方法。碳热合成在电弧炉中进行并且消耗大量能量,而铝热合成主要利用其自身的反应能量。因此,通过铝热合成,能量消耗下降。铝热合成是燃烧合成方法的一个子集,其名称基于在反应中用作还原剂的铝。在本研究中,研究了 NiB 的铝热合成条件(主要含有 15% 重量的 B)以增加反应物中 B2O3 的化学计量。此外,还进行了一些实验以了解镁热合成条件,作为替代还原剂。结果相互比较,并与使用 HSC Chemistry 6.12 和 FactSage 7.1 软件进行的热化学模拟进行比较。化学分析、SEM-EDS和XRD是表征原材料和产品的主要技术。增加 B2O3 化学计量和使用 Mg 作为还原剂对获得的 NiB 相的化学含量有积极影响,尽管镁热实验的金属回收率低于铝热实验的金属回收率。例如,在以 100% B2O3 化学计量进行的镁热实验中,获得的金属部分由 84.2% Ni、15.1% B 和 0.1% Mg 组成。化学分析、SEM-EDS和XRD是表征原材料和产品的主要技术。增加 B2O3 化学计量和使用 Mg 作为还原剂对获得的 NiB 相的化学含量有积极影响,尽管镁热实验的金属回收率低于铝热实验的金属回收率。例如,在以 100% B2O3 化学计量进行的镁热实验中,获得的金属部分由 84.2% Ni、15.1% B 和 0.1% Mg 组成。化学分析、SEM-EDS和XRD是表征原材料和产品的主要技术。增加 B2O3 化学计量和使用 Mg 作为还原剂对获得的 NiB 相的化学含量有积极影响,尽管镁热实验的金属回收率低于铝热实验的金属回收率。例如,在以 100% B2O3 化学计量进行的镁热实验中,获得的金属部分由 84.2% Ni、15.1% B 和 0.1% Mg 组成。
更新日期:2021-01-01
中文翻译:

还原剂类型对NiB燃烧合成的影响
摘要 Ni-B金属间合金是重要的钎焊材料。碳热和铝热合成路线是生产 NiB 的主要方法。碳热合成在电弧炉中进行并且消耗大量能量,而铝热合成主要利用其自身的反应能量。因此,通过铝热合成,能量消耗下降。铝热合成是燃烧合成方法的一个子集,其名称基于在反应中用作还原剂的铝。在本研究中,研究了 NiB 的铝热合成条件(主要含有 15% 重量的 B)以增加反应物中 B2O3 的化学计量。此外,还进行了一些实验以了解镁热合成条件,作为替代还原剂。结果相互比较,并与使用 HSC Chemistry 6.12 和 FactSage 7.1 软件进行的热化学模拟进行比较。化学分析、SEM-EDS和XRD是表征原材料和产品的主要技术。增加 B2O3 化学计量和使用 Mg 作为还原剂对获得的 NiB 相的化学含量有积极影响,尽管镁热实验的金属回收率低于铝热实验的金属回收率。例如,在以 100% B2O3 化学计量进行的镁热实验中,获得的金属部分由 84.2% Ni、15.1% B 和 0.1% Mg 组成。化学分析、SEM-EDS和XRD是表征原材料和产品的主要技术。增加 B2O3 化学计量和使用 Mg 作为还原剂对获得的 NiB 相的化学含量有积极影响,尽管镁热实验的金属回收率低于铝热实验的金属回收率。例如,在以 100% B2O3 化学计量进行的镁热实验中,获得的金属部分由 84.2% Ni、15.1% B 和 0.1% Mg 组成。化学分析、SEM-EDS和XRD是表征原材料和产品的主要技术。增加 B2O3 化学计量和使用 Mg 作为还原剂对获得的 NiB 相的化学含量有积极影响,尽管镁热实验的金属回收率低于铝热实验的金属回收率。例如,在以 100% B2O3 化学计量进行的镁热实验中,获得的金属部分由 84.2% Ni、15.1% B 和 0.1% Mg 组成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号