当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Formation of 2-Substituted 2H-Indazoles by a Pd-Catalyzed Reaction between 2-Halobenzyl Halides and Arylhydrazines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-12-11 , DOI: 10.1021/acs.joc.0c01923 Nayyef Aljaar 1 , Mousa Al-Noaimi 1 , Jürgen Conrad 2 , Uwe Beifuss 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-12-11 , DOI: 10.1021/acs.joc.0c01923 Nayyef Aljaar 1 , Mousa Al-Noaimi 1 , Jürgen Conrad 2 , Uwe Beifuss 2
Affiliation
|
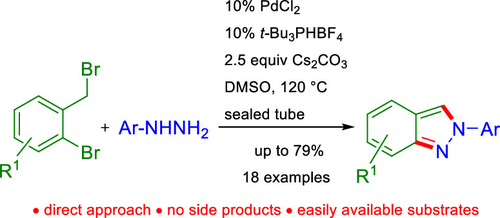
A direct and operationally simple method for the regioselective synthesis of 2-aryl-substituted 2H-indazoles is reported. The Pd-catalyzed reaction between easily available 2-bromobenzyl bromides and arylhydrazines employing Cs2CO3 as the base and t-Bu3PHBF4 as the ligand in DMSO at 120 °C in a sealed tube delivers the 2-substituted-2H-indazoles in a single synthetic step with yields up to 79%. The new method is based on a regioselective intermolecular N-benzylation followed by intramolecular N-arylation and oxidation.
中文翻译:

通过2-卤代苄基卤化物与芳肼之间的钯催化反应直接形成2-取代的2 H-吲唑
报道了直接和操作简单的方法用于区域选择性合成2-芳基取代的2 H-吲唑。容易获得的2-溴苄基溴化物和芳基肼采用铯之间的Pd-催化反应2 CO 3作为碱和吨-Bu 3 PHBF 4作为在密封管中120℃下在DMSO中配体递送2-取代- 2 ħ -吲唑在单个合成步骤中的收率高达79%。该新方法基于区域选择性的分子间N-苄基化,然后进行分子内N-芳基化和氧化。
更新日期:2021-01-16
中文翻译:

通过2-卤代苄基卤化物与芳肼之间的钯催化反应直接形成2-取代的2 H-吲唑
报道了直接和操作简单的方法用于区域选择性合成2-芳基取代的2 H-吲唑。容易获得的2-溴苄基溴化物和芳基肼采用铯之间的Pd-催化反应2 CO 3作为碱和吨-Bu 3 PHBF 4作为在密封管中120℃下在DMSO中配体递送2-取代- 2 ħ -吲唑在单个合成步骤中的收率高达79%。该新方法基于区域选择性的分子间N-苄基化,然后进行分子内N-芳基化和氧化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号