当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C–N, C–C, and C–O Bonds
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-12-10 , DOI: 10.1021/acs.chemrev.0c00803 Rémi Blieck 1 , Marc Taillefer 1 , Florian Monnier 1, 2
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-12-10 , DOI: 10.1021/acs.chemrev.0c00803 Rémi Blieck 1 , Marc Taillefer 1 , Florian Monnier 1, 2
Affiliation
|
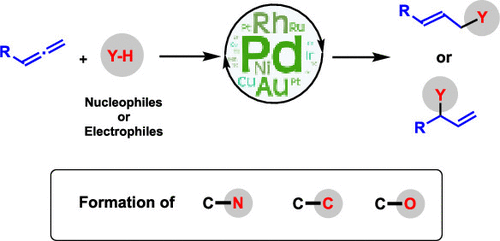
Following a strong regain of interest over the past 20 years in the chemistry of allenes, this “forgotten” family of unsaturated molecules is undergoing a renaissance. In this context, the metal-catalyzed hydrofunctionalization of allenes is nowadays one of the most studied transformations. The latter is of great interest because it opens a way to produce selectively functionalized allylic structures. These motifs are important in synthesis, particularly for the formation of asymmetric centers. Hydrofunctionalization of allenes is also a totally atom economical strategy, avoiding generation of any waste, to produce allylic functionalized structures. Compared to the main pathway to obtain the latter (aka Tsuji-Trost allylic substitution), metal-catalyzed hydrofunctionalization does not require the prefunctionalization of starting material with a leaving group. This review presents a state of the art exploration of all existing transition metal-catalyzed methods allowing the selective intermolecular hydrofunctionalization of allenes with N–H, C–H, and O–H nucleophiles or electrophiles.
中文翻译:

金属催化的丙二烯分子间氢官能化:通过C–N,CC–和C–O键的选择性形成,易于获得烯丙基结构
在过去的20年中,人们对艾伦的化学产生了浓厚的兴趣,此“被遗忘”的不饱和分子家族正在复兴。在这种情况下,如今,金属催化的丙二烯加氢官能化已成为研究最多的转化之一。后者引起了极大的兴趣,因为它为生产选择性功能化的烯丙基结构开辟了道路。这些基序在合成中很重要,特别是对于不对称中心的形成而言。丙二烯的加氢官能化也是完全原子经济的策略,避免产生任何废物,以产生烯丙基官能化的结构。与获得后者的主要途径(也称为Tsuji-Trost烯丙基取代)相比,金属催化的加氢官能化不需要具有离去基团的原料的预官能化。这篇综述提出了对所有现有的过渡金属催化方法的最新研究进展,这些方法允许用NH,CH和OH亲核试剂或亲电试剂选择性地进行烯丙基的分子间加氢官能化。
更新日期:2020-12-23
中文翻译:

金属催化的丙二烯分子间氢官能化:通过C–N,CC–和C–O键的选择性形成,易于获得烯丙基结构
在过去的20年中,人们对艾伦的化学产生了浓厚的兴趣,此“被遗忘”的不饱和分子家族正在复兴。在这种情况下,如今,金属催化的丙二烯加氢官能化已成为研究最多的转化之一。后者引起了极大的兴趣,因为它为生产选择性功能化的烯丙基结构开辟了道路。这些基序在合成中很重要,特别是对于不对称中心的形成而言。丙二烯的加氢官能化也是完全原子经济的策略,避免产生任何废物,以产生烯丙基官能化的结构。与获得后者的主要途径(也称为Tsuji-Trost烯丙基取代)相比,金属催化的加氢官能化不需要具有离去基团的原料的预官能化。这篇综述提出了对所有现有的过渡金属催化方法的最新研究进展,这些方法允许用NH,CH和OH亲核试剂或亲电试剂选择性地进行烯丙基的分子间加氢官能化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号