当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydroaminoalkylation/Buchwald‐Hartwig Amination Sequences for the Synthesis of Novel Thieno‐ or Benzothieno‐Annulated Tetrahydropyridines, Tetrahydroazasilines, and Tetrahydroazasilepines
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-12-11 , DOI: 10.1002/ejoc.202001523 Michael Warsitz 1 , Stefan H. Rohjans 1 , Marc Schmidtmann 1 , Sven Doye 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-12-11 , DOI: 10.1002/ejoc.202001523 Michael Warsitz 1 , Stefan H. Rohjans 1 , Marc Schmidtmann 1 , Sven Doye 1
Affiliation
|
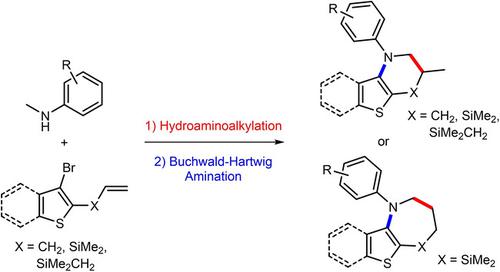
Hydroaminoalkylation reactions of 2‐allyl‐, 2‐allyldimethylsilyl‐, or 2‐dimethyl(vinyl)silyl‐substituted 3‐bromothiophenes or 3‐bromobenzothiophenes with secondary amines deliver the branched or the linear addition products with high regioselectivity. A combination of this reaction with a subsequent intramolecular Buchwald‐Hartwig amination results in several one‐pot processes that give direct access to structurally novel thiophene‐ or benzothiophene‐annulated 6‐ and 7‐membered heterocycles.

中文翻译:

合成新型硫代或苯并噻吩并环化的四氢吡啶,四氢氮杂lines啶和四氢氮杂阿司匹林的氢氨基烷基化/布赫瓦尔德-哈特维格胺胺化序列
2-仲烯丙基,2-烯丙基二甲基甲硅烷基或2-二甲基(乙烯基)甲硅烷基取代的3-溴噻吩或3-溴苯并噻吩与仲胺的加氢氨基烷基化反应可提供具有高区域选择性的支链或线性加成产物。该反应与随后的分子内Buchwald-Hartwig胺化反应相结合,产生了多个一锅法,可直接进入结构新颖的噻吩或苯并噻吩环化的6元和7元杂环。

更新日期:2021-02-01

中文翻译:

合成新型硫代或苯并噻吩并环化的四氢吡啶,四氢氮杂lines啶和四氢氮杂阿司匹林的氢氨基烷基化/布赫瓦尔德-哈特维格胺胺化序列
2-仲烯丙基,2-烯丙基二甲基甲硅烷基或2-二甲基(乙烯基)甲硅烷基取代的3-溴噻吩或3-溴苯并噻吩与仲胺的加氢氨基烷基化反应可提供具有高区域选择性的支链或线性加成产物。该反应与随后的分子内Buchwald-Hartwig胺化反应相结合,产生了多个一锅法,可直接进入结构新颖的噻吩或苯并噻吩环化的6元和7元杂环。


























 京公网安备 11010802027423号
京公网安备 11010802027423号