当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
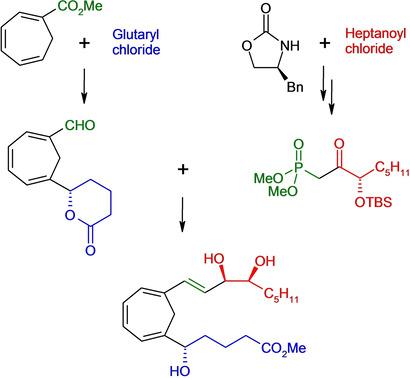
Synthesis of Enantiopure 6,11‐Methylene Lipoxin B4 Methyl Ester
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-12-11 , DOI: 10.1002/ejoc.202001591 Lukas Trippe 1 , Analuisa Nava 2 , Andrea Frank 1 , Udo Nubbemeyer 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-12-11 , DOI: 10.1002/ejoc.202001591 Lukas Trippe 1 , Analuisa Nava 2 , Andrea Frank 1 , Udo Nubbemeyer 1
Affiliation
|
A flexible convergent enantioselective total synthesis of a rigid lipoxin B4 analog incorporating a 6, 11‐methylene bridge has been developed. C1–C12 aldehyde and C13–C20 ketophosphonate underwent highly efficient key Horner olefination. A final six‐step sequence delivered the target compound displaying all stereogenic centers with standard lipoxin B4 configurations.

中文翻译:

对映纯6,11-亚甲基脂氧蛋白B4甲基酯的合成
已经开发了一种灵活的收敛性对映体选择性合成的硬脂蛋白B 4类似物,其中包含6、11-亚甲基桥。C1-C12醛和C13-C20酮膦酸酯进行了高效的关键霍纳烯化反应。最后的六步序列提供了目标化合物,该化合物显示了所有具有标准脂蛋白B 4构型的立体异构中心。

更新日期:2021-02-16

中文翻译:

对映纯6,11-亚甲基脂氧蛋白B4甲基酯的合成
已经开发了一种灵活的收敛性对映体选择性合成的硬脂蛋白B 4类似物,其中包含6、11-亚甲基桥。C1-C12醛和C13-C20酮膦酸酯进行了高效的关键霍纳烯化反应。最后的六步序列提供了目标化合物,该化合物显示了所有具有标准脂蛋白B 4构型的立体异构中心。




























 京公网安备 11010802027423号
京公网安备 11010802027423号