当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical research of second generation molecular motor with unidirectional rotary motion
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-12-08 , DOI: 10.1002/poc.4175 Qiuping Guan 1 , Hailong Wang 1 , Xueye Wang 1
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-12-08 , DOI: 10.1002/poc.4175 Qiuping Guan 1 , Hailong Wang 1 , Xueye Wang 1
Affiliation
|
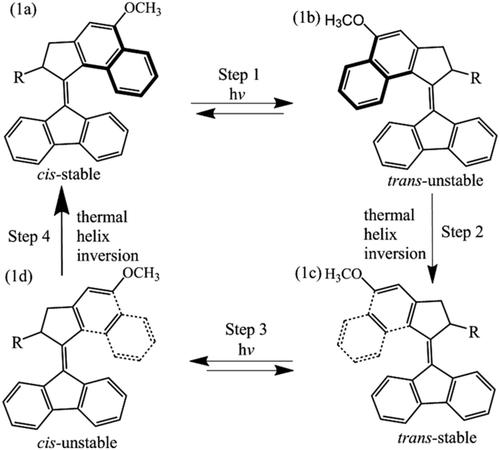
The rotational process and conformation inversion of the second generation molecular motor (9‐(5‐methoxy‐2‐methyl‐2, 3‐dihydro‐1H‐cyclopentyl[a]naphthalene‐1‐subunit)‐9H‐fluorene) are calculated and analyzed by potential energy surface scanning at the level of density functional theory, the M06‐2X functional combined with the def‐TZVP basis set. The effects of four different donor–acceptor substituents on the molecular stability are mainly explored. The fully optimized geometries of molecular motors 1–5 are discussed at the M06‐2X/def‐TZVP theoretical level. The energy barrier analysis of molecular motors shows that the nature of the substituents will not have a significant impact on the thermal isomerization barrier of the motor. Theoretical analysis of frontier molecular orbital (FMO) shows that after replacing the methyl group with phenyl, methoxy, fluorine, and cyano, the stability of the molecule is reduced. It can be seen from the absorption spectrum that the substituent makes the molecular motor absorption peak significantly red shifted from the absorption spectrum. The study of nuclear magnetic resonance (NMR) shows that it may be affected by deshielding effects, and the substitution of the methyl substituent causes most of the chemical shift (δ) of the molecular motor to a downfield. Finally, it is verified that the conformation of the motors changed from a stable state to unstable state during the photoisomerization process. The calculated results have explained the rotation of molecular motor well and can be used in the design of molecular motor.
中文翻译:

单向旋转运动第二代分子电动机的理论研究
第二代分子马达的旋转过程和构象反转(9-(5-甲氧基-2-甲基-2,3-二氢- 1 H ^ -环戊基[一个]萘-1-亚基)-9 ħ -芴)是M06-2X功能与def-TZVP基集相结合,通过在密度泛函理论水平上的势能表面扫描计算和分析。主要探讨了四种不同的供体-受体取代基对分子稳定性的影响。分子马达的完全优化构1 - 5在M06-2X / def-TZVP理论水平上进行了讨论。分子电动机的能垒分析表明,取代基的性质不会对电动机的热异构化障碍产生重大影响。前沿分子轨道(FMO)的理论分析表明,用苯基,甲氧基,氟和氰基取代甲基后,分子的稳定性降低。从吸收光谱可以看出,取代基使分子马达吸收峰从吸收光谱显着红移。对核磁共振(NMR)的研究表明,它可能受到屏蔽作用的影响,而甲基取代基的取代会引起大部分化学位移(δ)的分子马达到低场。最后,证实了在光异构化过程中电动机的构型从稳定状态变为不稳定状态。计算结果很好地说明了分子电动机的旋转,可用于分子电动机的设计。
更新日期:2020-12-08
中文翻译:

单向旋转运动第二代分子电动机的理论研究
第二代分子马达的旋转过程和构象反转(9-(5-甲氧基-2-甲基-2,3-二氢- 1 H ^ -环戊基[一个]萘-1-亚基)-9 ħ -芴)是M06-2X功能与def-TZVP基集相结合,通过在密度泛函理论水平上的势能表面扫描计算和分析。主要探讨了四种不同的供体-受体取代基对分子稳定性的影响。分子马达的完全优化构1 - 5在M06-2X / def-TZVP理论水平上进行了讨论。分子电动机的能垒分析表明,取代基的性质不会对电动机的热异构化障碍产生重大影响。前沿分子轨道(FMO)的理论分析表明,用苯基,甲氧基,氟和氰基取代甲基后,分子的稳定性降低。从吸收光谱可以看出,取代基使分子马达吸收峰从吸收光谱显着红移。对核磁共振(NMR)的研究表明,它可能受到屏蔽作用的影响,而甲基取代基的取代会引起大部分化学位移(δ)的分子马达到低场。最后,证实了在光异构化过程中电动机的构型从稳定状态变为不稳定状态。计算结果很好地说明了分子电动机的旋转,可用于分子电动机的设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号