Chem ( IF 23.5 ) Pub Date : 2020-12-09 , DOI: 10.1016/j.chempr.2020.11.017 Tyler P Pabst 1 , Linda Quach 1 , Kaitlyn T MacMillan 1 , Paul J Chirik 1, 2
|
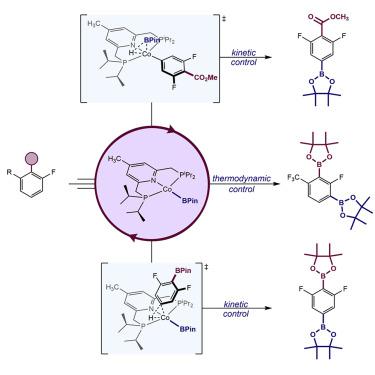
Synthetic and mechanistic investigations into the C(sp2)-H borylation of various electronically diverse arenes catalyzed by bis(phosphine)pyridine (iPrPNP) cobalt complexes are reported. Borylation of various benzoate esters and arylboronate esters gave remarkably high selectivities for the position para to the functional group; in both cases, this regioselectivity was found to override the ortho-to-fluorine regioselectivity, previously reported for (iPrPNP)Co borylation catalysts, which arises from thermodynamic control of C(sp2)-H oxidative addition. Mechanistic studies support pathways that result in para-to-ester and para-to-boronate ester selectivity by kinetic control of B-H and C(sp2-H) oxidative addition, respectively. Borylation of a particularly electron-deficient fluorinated arylboronate ester resulted in acceleration of C(sp2)-H oxidative addition and concomitant inversion of regioselectivity, demonstrating that subtle changes in the relative rates of individual steps of the catalytic cycle can enable unique and switchable site selectivities.
中文翻译:

钴催化苯甲酸酯和芳基硼酸酯的 C(sp2)-H 硼化反应中区域选择性的机理起源
报道了双(膦)吡啶 ( iPr PNP) 钴配合物催化的各种电子多样性芳烃的 C(sp 2 )-H 硼化反应的合成和机理研究。各种苯甲酸酯和芳基硼酸酯的硼化反应对官能团的对位产生了非常高的选择性;在这两种情况下,发现这种区域选择性覆盖了之前报道的 ( iPr PNP)Co 硼化催化剂的邻位到氟的区域选择性,这是由 C(sp 2 )-H 氧化加成的热力学控制引起的。机理研究支持导致对位到酯和对位的途径分别通过动力学控制 BH 和 C(sp 2 -H) 氧化加成对硼酸酯的选择性。一种特别缺电子的氟化芳基硼酸酯的硼化导致 C(sp 2 )-H 氧化加成的加速和伴随的区域选择性反转,表明催化循环各个步骤的相对速率的细微变化可以实现独特且可切换的位点选择性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号