当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improving Thermodynamic Stability and Anticoagulant Activity of a Thrombin Binding Aptamer by Incorporation of 8-trifluoromethyl-2′-deoxyguanosine
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.jmedchem.0c01711 Hong-Liang Bao 1 , Takumi Ishizuka 1 , Atsushi Yamashita 2 , Eiji Furukoji 3 , Yujiro Asada 2 , Yan Xu 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.jmedchem.0c01711 Hong-Liang Bao 1 , Takumi Ishizuka 1 , Atsushi Yamashita 2 , Eiji Furukoji 3 , Yujiro Asada 2 , Yan Xu 1
Affiliation
|
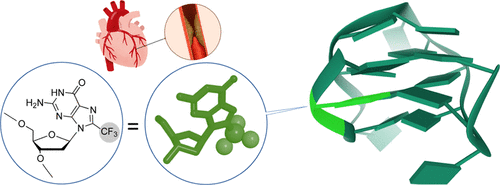
In this study, we incorporated 8-trifluoromethyl-2′-deoxyguanosine (FG) into a thrombin binding aptamer (TBA). Circular dichroism, nuclear magnetic resonance (NMR), electrophoresis, and prothrombin time (PT) assay were performed to investigate the structure, thermodynamic stability, biological stability, and anticoagulant activity of the FG-modified TBA sequences. We found that the replacement of FG into TBA sequences led to a remarkable improvement in the melting temperature up to 30 °C compared with the native sequence. The trifluoromethyl group allowed us to investigate the TBA G-quadruplex structure by 19F NMR spectroscopy. Furthermore, PT assays showed that the modified sequences can significantly improve the anticoagulant activity in comparison with the native TBA. Finally, we demonstrated that the trifluoromethyl-modified TBA sequence could function as an anticoagulant reagent in live rats. Our results strongly suggested that FG is a powerful nucleoside derivative to increase the thermodynamic stability and anticoagulant activity of TBA.
中文翻译:

通过掺入8-三氟甲基-2'-脱氧鸟苷提高凝血酶结合适体的热力学稳定性和抗凝活性。
在这项研究中,我们将8-三氟甲基-2'-脱氧鸟苷(F G)掺入凝血酶结合适体(TBA)中。进行了圆二色性,核磁共振(NMR),电泳和凝血酶原时间(PT)分析,以研究F G修饰的TBA序列的结构,热力学稳定性,生物学稳定性和抗凝血活性。我们发现,与天然序列相比,将F G替换为TBA序列可导致最高30°C的解链温度显着提高。三氟甲基使我们能够在19年前研究TBA G-四链体结构F NMR光谱。此外,PT测定显示与天然TBA相比,修饰的序列可以显着改善抗凝活性。最后,我们证明了三氟甲基修饰的TBA序列可以在活大鼠中充当抗凝剂。我们的结果强烈表明,F G是强大的核苷衍生物,可提高TBA的热力学稳定性和抗凝活性。
更新日期:2021-01-14
中文翻译:

通过掺入8-三氟甲基-2'-脱氧鸟苷提高凝血酶结合适体的热力学稳定性和抗凝活性。
在这项研究中,我们将8-三氟甲基-2'-脱氧鸟苷(F G)掺入凝血酶结合适体(TBA)中。进行了圆二色性,核磁共振(NMR),电泳和凝血酶原时间(PT)分析,以研究F G修饰的TBA序列的结构,热力学稳定性,生物学稳定性和抗凝血活性。我们发现,与天然序列相比,将F G替换为TBA序列可导致最高30°C的解链温度显着提高。三氟甲基使我们能够在19年前研究TBA G-四链体结构F NMR光谱。此外,PT测定显示与天然TBA相比,修饰的序列可以显着改善抗凝活性。最后,我们证明了三氟甲基修饰的TBA序列可以在活大鼠中充当抗凝剂。我们的结果强烈表明,F G是强大的核苷衍生物,可提高TBA的热力学稳定性和抗凝活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号