当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinase Photoaffinity Labeling Reveals Low Selectivity Profile of the IRE1 Targeting Imidazopyrazine-Based KIRA6 Inhibitor
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-12-08 , DOI: 10.1021/acschembio.0c00802 Dimitris Korovesis 1 , Nicole Rufo 2, 3 , Rita Derua 4, 5 , Patrizia Agostinis 2, 3 , Steven H L Verhelst 1, 6
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-12-08 , DOI: 10.1021/acschembio.0c00802 Dimitris Korovesis 1 , Nicole Rufo 2, 3 , Rita Derua 4, 5 , Patrizia Agostinis 2, 3 , Steven H L Verhelst 1, 6
Affiliation
|
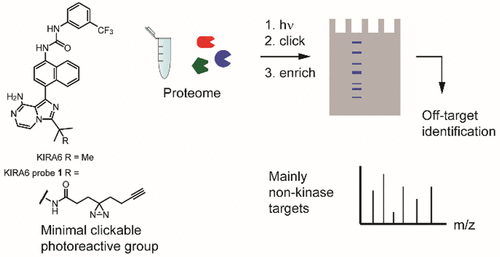
Inositol-requiring enzyme 1α (IRE1α) is one of three endoplasmic reticulum stress sensors. Upon activation of its kinase domain, IRE1α splices the mRNA substrate XBP1, which activates the unfolded protein response. IRE1α has emerged as a therapeutic target as its hyperactivation is implicated in various diseases. Kinase inhibiting RNase attenuator 6 (KIRA6) is an allosteric IRE1α inhibitor targeting the ATP binding pocket, resulting in effective blockage of the IRE1α-XBP1 pathway in mouse models of diabetes and pain. However, recent studies indicate that KIRA6 is not as selective as initially thought. Here, we developed a photoaffinity-based KIRA6 probe to reveal its selectivity. Surprisingly, the majority of off-targets that we identified were not protein kinases but mostly nucleotide-binding proteins. Furthermore, we found that the promiscuous off-target profile of KIRA6 is not cell-line-dependent. Overall, this study calls for caution when KIRA6 is used in IRE1α-targeted studies and illustrates the power of kinase photoaffinity probes.
中文翻译:

激酶光亲和标记揭示了IRE1靶向基于咪唑并吡嗪的KIRA6抑制剂的低选择性
需要肌醇的酶1α(IRE1α)是三个内质网应激传感器之一。激活其激酶结构域后,IRE1α剪接mRNA底物XBP1,从而激活未折叠的蛋白质反应。由于IRE1α的过度活化与多种疾病有关,因此它已成为一种治疗靶标。激酶抑制性RNase衰减器6(KIRA6)是靶向ATP结合袋的变构性IRE1α抑制剂,可在糖尿病和疼痛的小鼠模型中有效阻断IRE1α-XBP1途径。但是,最近的研究表明,KIRA6的选择性不如最初的想象。在这里,我们开发了一种基于光亲和力的KIRA6探针来揭示其选择性。令人惊讶的是,我们鉴定出的大多数脱靶分子不是蛋白激酶,而是核苷酸结合蛋白。此外,我们发现,KIRA6的杂散脱靶谱与细胞系无关。总体而言,这项研究在IRE1α靶向研究中使用KIRA6时需要引起注意,并说明了激酶光亲和探针的功能。
更新日期:2020-12-18
中文翻译:

激酶光亲和标记揭示了IRE1靶向基于咪唑并吡嗪的KIRA6抑制剂的低选择性
需要肌醇的酶1α(IRE1α)是三个内质网应激传感器之一。激活其激酶结构域后,IRE1α剪接mRNA底物XBP1,从而激活未折叠的蛋白质反应。由于IRE1α的过度活化与多种疾病有关,因此它已成为一种治疗靶标。激酶抑制性RNase衰减器6(KIRA6)是靶向ATP结合袋的变构性IRE1α抑制剂,可在糖尿病和疼痛的小鼠模型中有效阻断IRE1α-XBP1途径。但是,最近的研究表明,KIRA6的选择性不如最初的想象。在这里,我们开发了一种基于光亲和力的KIRA6探针来揭示其选择性。令人惊讶的是,我们鉴定出的大多数脱靶分子不是蛋白激酶,而是核苷酸结合蛋白。此外,我们发现,KIRA6的杂散脱靶谱与细胞系无关。总体而言,这项研究在IRE1α靶向研究中使用KIRA6时需要引起注意,并说明了激酶光亲和探针的功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号