当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding the Hydrogen Evolution Reaction Kinetics of Electrodeposited Nickel‐Molybdenum in Acidic, Near‐Neutral, and Alkaline Conditions
ChemElectroChem ( IF 4 ) Pub Date : 2020-12-08 , DOI: 10.1002/celc.202001436 Fuxi Bao 1 , Erno Kemppainen 1 , Iris Dorbandt 1 , Radu Bors 1 , Fanxing Xi 1 , Rutger Schlatmann 1 , Roel Krol 2 , Sonya Calnan 1
ChemElectroChem ( IF 4 ) Pub Date : 2020-12-08 , DOI: 10.1002/celc.202001436 Fuxi Bao 1 , Erno Kemppainen 1 , Iris Dorbandt 1 , Radu Bors 1 , Fanxing Xi 1 , Rutger Schlatmann 1 , Roel Krol 2 , Sonya Calnan 1
Affiliation
|
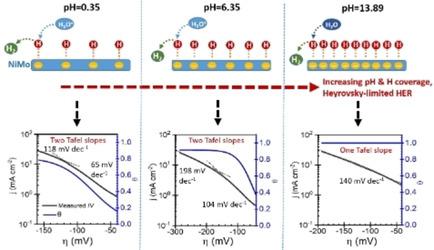
Nickel‐molybdenum (NiMo) alloys can be a possible alternative to platinum as hydrogen evolution reaction (HER) catalysts because of the superior HER activity. However, the superior HER activity and the pH‐dependent kinetics are not currently fully understood. Herein, we present a study of HER kinetics and mechanisms of NiMo in alkaline, near‐neutral and acidic media by combining voltammetry measurements with electrochemical impedance spectroscopy and a microkinetic model. The results indicate that, compared to Ni, NiMo has significantly higher active surface area and intrinsic HER activity. In the subsequent measurements, we demonstrated that different from the existing explanations to the HER mechanisms for NiMo, the HER process in acidic, near‐neutral, and alkaline media is controlled by the Heyrovsky step. Our results show that increasing pH increases the hydrogen coverage, which increases the Tafel‐slope at low overpotentials, eventually resulting in only a single Tafel slope, which would commonly be interpreted as a Volmer‐limited reaction. Furthermore, the studies of thickness effect on HER kinetics show that the HER kinetics of NiMo are thickness‐dependent. In phosphate buffer, the increase in thickness did not significantly increase the double‐layer capacitance, but simulations with the microkinetic model indicate that the active surface area still increased similarly to other electrolytes, which is likely related to the type of electrolyte used.
中文翻译:

了解酸性,近中性和碱性条件下电沉积镍钼的氢析出反应动力学
镍钼(NiMo)合金由于其优异的HER活性,可以作为铂作为氢释放反应(HER)催化剂的替代品。但是,目前尚不完全清楚其优异的HER活性和pH依赖性动力学。在这里,我们通过结合伏安法测量与电化学阻抗谱和微动力学模型,研究了在碱性,近中性和酸性介质中NiMo的HER动力学和机理。结果表明,与Ni相比,NiMo具有显着更高的活性表面积和固有的HER活性。在随后的测量中,我们证明了与现有对NiMo的HER机制的解释不同,在酸性,接近中性和碱性介质中的HER过程受Heyrovsky步骤控制。我们的结果表明,增加pH值会增加氢的覆盖率,从而在低超电势下增加Tafel斜率,最终仅导致一个Tafel斜率,这通常被解释为Volmer限制的反应。此外,厚度对HER动力学的影响研究表明,NiMo的HER动力学与厚度有关。在磷酸盐缓冲液中,厚度的增加并没有显着增加双层电容,但是通过微动力学模型进行的模拟表明,活性表面积仍然与其他电解质类似地增加,这可能与所用电解质的类型有关。此外,厚度对HER动力学的影响研究表明,NiMo的HER动力学与厚度有关。在磷酸盐缓冲液中,厚度的增加并没有显着增加双层电容,但是通过微动力学模型进行的模拟表明,活性表面积仍然与其他电解质类似地增加,这可能与所用电解质的类型有关。此外,厚度对HER动力学的影响研究表明,NiMo的HER动力学与厚度有关。在磷酸盐缓冲液中,厚度的增加并没有显着增加双层电容,但是通过微动力学模型进行的模拟表明,活性表面积仍然与其他电解质类似地增加,这可能与所用电解质的类型有关。
更新日期:2021-01-05
中文翻译:

了解酸性,近中性和碱性条件下电沉积镍钼的氢析出反应动力学
镍钼(NiMo)合金由于其优异的HER活性,可以作为铂作为氢释放反应(HER)催化剂的替代品。但是,目前尚不完全清楚其优异的HER活性和pH依赖性动力学。在这里,我们通过结合伏安法测量与电化学阻抗谱和微动力学模型,研究了在碱性,近中性和酸性介质中NiMo的HER动力学和机理。结果表明,与Ni相比,NiMo具有显着更高的活性表面积和固有的HER活性。在随后的测量中,我们证明了与现有对NiMo的HER机制的解释不同,在酸性,接近中性和碱性介质中的HER过程受Heyrovsky步骤控制。我们的结果表明,增加pH值会增加氢的覆盖率,从而在低超电势下增加Tafel斜率,最终仅导致一个Tafel斜率,这通常被解释为Volmer限制的反应。此外,厚度对HER动力学的影响研究表明,NiMo的HER动力学与厚度有关。在磷酸盐缓冲液中,厚度的增加并没有显着增加双层电容,但是通过微动力学模型进行的模拟表明,活性表面积仍然与其他电解质类似地增加,这可能与所用电解质的类型有关。此外,厚度对HER动力学的影响研究表明,NiMo的HER动力学与厚度有关。在磷酸盐缓冲液中,厚度的增加并没有显着增加双层电容,但是通过微动力学模型进行的模拟表明,活性表面积仍然与其他电解质类似地增加,这可能与所用电解质的类型有关。此外,厚度对HER动力学的影响研究表明,NiMo的HER动力学与厚度有关。在磷酸盐缓冲液中,厚度的增加并没有显着增加双层电容,但是通过微动力学模型进行的模拟表明,活性表面积仍然与其他电解质类似地增加,这可能与所用电解质的类型有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号