Structure ( IF 5.7 ) Pub Date : 2020-12-08 , DOI: 10.1016/j.str.2020.11.014 Hsiang-Ting Lei 1 , Xuelang Mu 2 , Johan Hattne 3 , Tamir Gonen 2
|
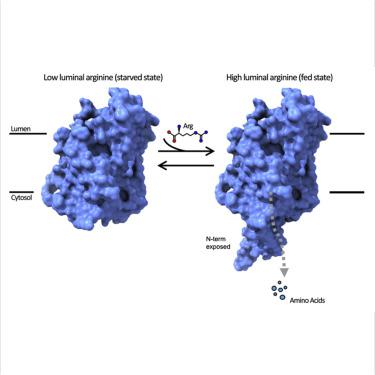
mTORC1 is a central hub that integrates environmental cues, such as cellular stresses and nutrient availability to modulate metabolism and cellular responses. Recently, SLC38A9, a lysosomal amino acid transporter, emerged as a sensor for luminal arginine and as an activator of mTORC1. The amino acid-mediated activation of mTORC1 is regulated by the N-terminal domain of SLC38A9. Here, we determined the crystal structure of zebrafish SLC38A9 (drSLC38A9) and found the N-terminal fragment inserted deep within the transporter, bound in the substrate-binding pocket where normally arginine would bind. This represents a significant conformational change of the N-terminal domain (N-plug) when compared with our recent arginine-bound structure of drSLC38A9. We propose a ball-and-chain model for mTORC1 activation, where N-plug insertion and Rag GTPase binding with SLC38A9 is regulated by luminal arginine levels. This work provides important insights into nutrient sensing by SLC38A9 to activate the mTORC1 pathways in response to dietary amino acids.
中文翻译:

SLC38A9 N 末端的构象变化表明 mTORC1 激活
mTORC1 是一个中央枢纽,它整合了环境线索,例如细胞压力和营养可用性,以调节新陈代谢和细胞反应。最近,溶酶体氨基酸转运蛋白 SLC38A9 作为管腔精氨酸传感器和 mTORC1 激活剂出现。氨基酸介导的 mTORC1 激活受 SLC38A9 的 N 末端结构域调节。在这里,我们确定了斑马鱼 SLC38A9 (drSLC38A9) 的晶体结构,并发现 N 末端片段插入转运体深处,结合在通常精氨酸结合的底物结合袋中。与我们最近的 drSLC38A9 精氨酸结合结构相比,这代表了 N 末端结构域(N-plug)的显着构象变化。我们提出了一个用于 mTORC1 激活的球链模型,其中 N-plug 插入和 Rag GTPase 与 SLC38A9 的结合受管腔精氨酸水平的调节。这项工作为 SLC38A9 的营养感应提供了重要的见解,以激活 mTORC1 通路以响应膳食氨基酸。

























 京公网安备 11010802027423号
京公网安备 11010802027423号