当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Porous Iron–Zirconium–Zinc Ternary Metal Oxide Scaffold: Facile Synthesis and Efficient Removal of Malachite Green from Water
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-06 , DOI: 10.1021/acs.jced.0c00681 Jhilirani Mohanta 1 , Roshni Kumari 1 , Banashree Dey 2 , Soumen Dey 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-06 , DOI: 10.1021/acs.jced.0c00681 Jhilirani Mohanta 1 , Roshni Kumari 1 , Banashree Dey 2 , Soumen Dey 1
Affiliation
|
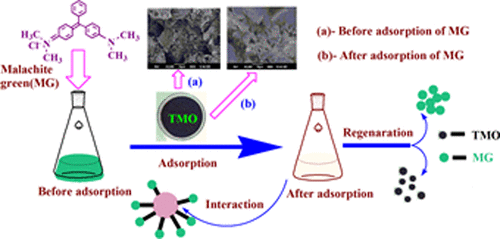
Designing water-stable and sustainable adsorbents for wastewater management has gained significant importance nowadays. This paper reports coprecipitation-induced rapid synthesis of a highly porous ternary metal oxide (TMO), its characterization, and efficient removal of malachite green (MG) from simulated water by a batch method. The material was characterized by the Brunauer–Emmett–Teller (BET) surface area, scanning electron microscopy (SEM), powder X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, point of zero charge (pHzpc), and chemical analysis. With a high surface area of 185.04 m2/g, TMO offers an excellent adsorption capacity of 133 mg/g under optimized conditions. pH-dependent adsorption was seen, and maximum adsorption was attained at the neutral condition, i.e., pH 7. Intraparticle diffusion (R2 = 0.998) and a combined Langmuir and Freundlich isotherm model (R2 = 0.987–0.998) best describe the adsorption pathway, suggesting that the process undergoes diffusive mass transfer and multilayer adsorption. The adsorption mechanism is proposed to be a combination of electrostatic interaction and hydrogen bonding. Thermodynamic parameters suggest a spontaneous (ΔG, −3.335 kJ/mol), feasible, and exothermic (ΔH, −8.436 kJ/mol) process. The low activation energy (3.459 kJ/mol) suggests a physisorption process. The material can be regenerated up to 83% by 0.1 M hydrochloric acid and reused for up to four cycles without significant loss of activity.
中文翻译:

高度多孔的铁-锆-锌三元金属氧化物支架:孔雀石绿的简便合成和有效去除
如今,设计用于废水管理的水稳定性和可持续性吸附剂已变得越来越重要。本文报道了共沉淀诱导的高孔隙度三元金属氧化物(TMO)的快速合成,其表征以及通过分批方法从模拟水中有效去除孔雀石绿(MG)的过程。该材料的特征在于Brunauer-Emmett-Teller(BET)表面积,扫描电子显微镜(SEM),粉末X射线衍射(XRD),傅里叶变换红外(FTIR)光谱,零电荷点(pH zpc),和化学分析。具有185.04 m 2的高表面积/ g,在优化条件下,TMO具有133 mg / g的出色吸附能力。观察到pH依赖的吸附,并且在中性条件下,即pH 7,达到最大吸附。颗粒内扩散(R 2 = 0.998)以及Langmuir和Freundlich等温线模型(R 2 = 0.987-0.998)最能描述吸附途径,表明该过程经历了扩散传质和多层吸附。提出的吸附机理是静电相互作用和氢键结合的结果。热力学参数表明是自发的(ΔG,-3.335 kJ / mol),可行,放热的(ΔH,-8.436 kJ / mol)过程。低活化能(3.459 kJ / mol)表明存在物理吸附过程。该材料可通过0.1 M盐酸再生高达83%,并重复使用多达四个循环,而活性没有明显降低。
更新日期:2021-01-14
中文翻译:

高度多孔的铁-锆-锌三元金属氧化物支架:孔雀石绿的简便合成和有效去除
如今,设计用于废水管理的水稳定性和可持续性吸附剂已变得越来越重要。本文报道了共沉淀诱导的高孔隙度三元金属氧化物(TMO)的快速合成,其表征以及通过分批方法从模拟水中有效去除孔雀石绿(MG)的过程。该材料的特征在于Brunauer-Emmett-Teller(BET)表面积,扫描电子显微镜(SEM),粉末X射线衍射(XRD),傅里叶变换红外(FTIR)光谱,零电荷点(pH zpc),和化学分析。具有185.04 m 2的高表面积/ g,在优化条件下,TMO具有133 mg / g的出色吸附能力。观察到pH依赖的吸附,并且在中性条件下,即pH 7,达到最大吸附。颗粒内扩散(R 2 = 0.998)以及Langmuir和Freundlich等温线模型(R 2 = 0.987-0.998)最能描述吸附途径,表明该过程经历了扩散传质和多层吸附。提出的吸附机理是静电相互作用和氢键结合的结果。热力学参数表明是自发的(ΔG,-3.335 kJ / mol),可行,放热的(ΔH,-8.436 kJ / mol)过程。低活化能(3.459 kJ / mol)表明存在物理吸附过程。该材料可通过0.1 M盐酸再生高达83%,并重复使用多达四个循环,而活性没有明显降低。

























 京公网安备 11010802027423号
京公网安备 11010802027423号