当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cyclic peptides bearing the d‐Phe‐2‐Abz turn motif: Structural characterization and antimicrobial potential
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2020-12-06 , DOI: 10.1002/psc.3291 Kyriakos G Varnava 1 , Patrick J B Edwards 2, 3 , Alan J Cameron 1, 3 , Elena Harjes 2, 3 , Vijayalekshmi Sarojini 1, 4
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2020-12-06 , DOI: 10.1002/psc.3291 Kyriakos G Varnava 1 , Patrick J B Edwards 2, 3 , Alan J Cameron 1, 3 , Elena Harjes 2, 3 , Vijayalekshmi Sarojini 1, 4
Affiliation
|
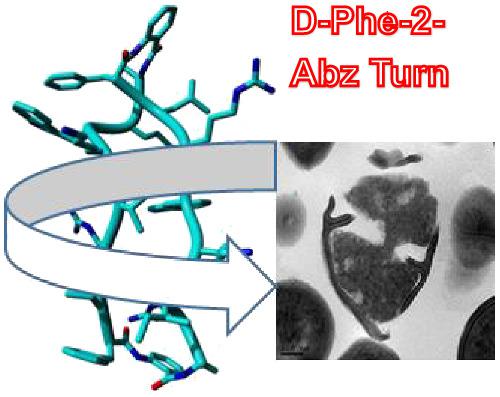
The effect on secondary structure and antimicrobial activity of introducing different cyclic constraints in linear β‐hairpin antimicrobial peptides has been investigated with the intention of generating cyclic β sheets as promising antimicrobials with improved therapeutic potential. The linear peptides were cyclized head to tail either directly or after the addition of either a second turn motif or a disulfide bridge. The propensity of these peptides to adopt a cyclic β‐sheet structure has been correlated to their antibacterial activity. All cyclic peptides showed enhanced activity, compared with their linear counterparts against methicillin‐resistant Staphylococcus aureus. Scanning electron microscopy and transmission electron microscopy studies showed that this family kills bacteria through membrane lysis. The peptide that showed the best efficacy against all strains (exhibiting nanomolar activity), while retaining low haemolysis, bears two symmetrical, homochiral d‐phe‐2‐Abz‐d‐ala turns and adopted a flexible structure. Its twin peptide that bears heterochiral turns (one with d‐ala and one with L‐Ala) showed reduced antibacterial activity and higher percentage of haemolysis. Circular dichroism and nuclear magnetic resonance spectroscopy indicate that heterochirality in the two turns leads to oligomerization of the peptide at higher concentrations, stabilizing the β‐sheet secondary structure. More rigid secondary structure is associated with lower activity against bacteria and loss of selectivity.
中文翻译:

带有 d-Phe-2-Abz 转角基序的环肽:结构表征和抗菌潜力
已经研究了在线性 β-发夹抗菌肽中引入不同环状约束对二级结构和抗菌活性的影响,目的是生成环状 β 折叠作为具有改善治疗潜力的有前途的抗菌剂。将线性肽直接或在添加第二个转角基序或二硫键后头对尾环化。这些肽采用环状β-折叠结构的倾向与其抗菌活性相关。与线性对应物相比,所有环肽对耐甲氧西林金黄色葡萄球菌均表现出增强的活性. 扫描电子显微镜和透射电子显微镜研究表明,该家族通过膜裂解杀死细菌。对所有菌株表现出最佳功效(表现出纳摩尔活性)的肽,在保持低溶血的同时,具有两个对称的同手性d -phe-2-Abz- d -ala转角并采用柔性结构。它带有异手性转角的双肽(一个带有d-ala 和含有 L-Ala 的一种)显示出抗菌活性降低和溶血百分比更高。圆二色性和核磁共振波谱表明,两圈中的异手性导致肽在较高浓度下寡聚化,稳定β-折叠二级结构。更刚性的二级结构与较低的细菌活性和选择性的丧失有关。
更新日期:2021-01-04
中文翻译:

带有 d-Phe-2-Abz 转角基序的环肽:结构表征和抗菌潜力
已经研究了在线性 β-发夹抗菌肽中引入不同环状约束对二级结构和抗菌活性的影响,目的是生成环状 β 折叠作为具有改善治疗潜力的有前途的抗菌剂。将线性肽直接或在添加第二个转角基序或二硫键后头对尾环化。这些肽采用环状β-折叠结构的倾向与其抗菌活性相关。与线性对应物相比,所有环肽对耐甲氧西林金黄色葡萄球菌均表现出增强的活性. 扫描电子显微镜和透射电子显微镜研究表明,该家族通过膜裂解杀死细菌。对所有菌株表现出最佳功效(表现出纳摩尔活性)的肽,在保持低溶血的同时,具有两个对称的同手性d -phe-2-Abz- d -ala转角并采用柔性结构。它带有异手性转角的双肽(一个带有d-ala 和含有 L-Ala 的一种)显示出抗菌活性降低和溶血百分比更高。圆二色性和核磁共振波谱表明,两圈中的异手性导致肽在较高浓度下寡聚化,稳定β-折叠二级结构。更刚性的二级结构与较低的细菌活性和选择性的丧失有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号