当前位置:
X-MOL 学术
›
Biol. Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Specialised endocytic proteins regulate diverse internalisation mechanisms and signalling outputs in physiology and cancer
Biology of the Cell ( IF 2.7 ) Pub Date : 2020-12-06 , DOI: 10.1111/boc.202000129 Giovanni Giangreco 1 , Maria Grazia Malabarba 2, 3 , Sara Sigismund 2, 3
Biology of the Cell ( IF 2.7 ) Pub Date : 2020-12-06 , DOI: 10.1111/boc.202000129 Giovanni Giangreco 1 , Maria Grazia Malabarba 2, 3 , Sara Sigismund 2, 3
Affiliation
|
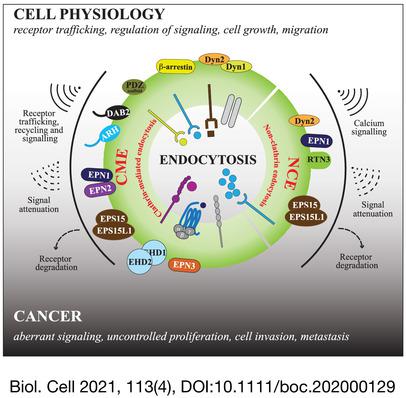
Although endocytosis was first described as the process mediating macromolecule or nutrient uptake through the plasma membrane, it is now recognised as a critical component of the cellular infrastructure involved in numerous processes, ranging from receptor signalling, proliferation and migration to polarity and stem cell regulation. To realise these varying roles, endocytosis needs to be finely regulated. Accordingly, multiple endocytic mechanisms exist that require specialised molecular machineries and an array of endocytic adaptor proteins with cell‐specific functions. This review provides some examples of specialised functions of endocytic adaptors and other components of the endocytic machinery in different cell physiological processes, and how the alteration of these functions is linked to cancer. In particular, we focus on: (i) cargo selection and endocytic mechanisms linked to different adaptors; (ii) specialised functions in clathrin‐mediated versus non‐clathrin endocytosis; (iii) differential regulation of endocytic mechanisms by post‐translational modification of endocytic proteins; (iv) cell context‐dependent expression and function of endocytic proteins. As cases in point, we describe two endocytic protein families, dynamins and epsins. Finally, we discuss how dysregulation of the physiological role of these specialised endocytic proteins is exploited by cancer cells to increase cell proliferation, migration and invasion, leading to anti‐apoptotic or pro‐metastatic behaviours.
中文翻译:

专门的内吞蛋白调节生理学和癌症中的多种内化机制和信号输出
尽管内吞作用最初被描述为介导大分子或通过质膜吸收营养物质的过程,但现在它被认为是细胞基础结构的关键组成部分,涉及从受体信号传导、增殖和迁移到极性和干细胞调节等众多过程。为了实现这些不同的作用,需要对内吞作用进行精细调节。因此,存在多种内吞机制,需要专门的分子机制和一系列具有细胞特异性功能的内吞衔接蛋白。这篇综述提供了一些内吞衔接子和内吞机制的其他组件在不同细胞生理过程中的特殊功能的例子,以及这些功能的改变如何与癌症相关联。特别是,我们专注于:(i) 与不同适配器相关的货物选择和内吞机制;(ii) 网格蛋白介导与非网格蛋白内吞作用的特殊功能;(iii) 通过内吞蛋白的翻译后修饰对内吞机制的差异调节;(iv) 内吞蛋白的细胞环境依赖性表达和功能。作为恰当的例子,我们描述了两个内吞蛋白家族,动力蛋白和 epsins。最后,我们讨论了癌细胞如何利用这些特殊内吞蛋白的生理作用失调来增加细胞增殖、迁移和侵袭,从而导致抗凋亡或促转移行为。(iii) 通过内吞蛋白的翻译后修饰对内吞机制的差异调节;(iv) 内吞蛋白的细胞环境依赖性表达和功能。作为恰当的例子,我们描述了两个内吞蛋白家族,动力蛋白和 epsins。最后,我们讨论了癌细胞如何利用这些特殊内吞蛋白的生理作用失调来增加细胞增殖、迁移和侵袭,从而导致抗凋亡或促转移行为。(iii) 通过内吞蛋白的翻译后修饰对内吞机制的差异调节;(iv) 内吞蛋白的细胞环境依赖性表达和功能。作为恰当的例子,我们描述了两个内吞蛋白家族,动力蛋白和 epsins。最后,我们讨论了癌细胞如何利用这些特殊内吞蛋白的生理作用失调来增加细胞增殖、迁移和侵袭,从而导致抗凋亡或促转移行为。
更新日期:2020-12-06
中文翻译:

专门的内吞蛋白调节生理学和癌症中的多种内化机制和信号输出
尽管内吞作用最初被描述为介导大分子或通过质膜吸收营养物质的过程,但现在它被认为是细胞基础结构的关键组成部分,涉及从受体信号传导、增殖和迁移到极性和干细胞调节等众多过程。为了实现这些不同的作用,需要对内吞作用进行精细调节。因此,存在多种内吞机制,需要专门的分子机制和一系列具有细胞特异性功能的内吞衔接蛋白。这篇综述提供了一些内吞衔接子和内吞机制的其他组件在不同细胞生理过程中的特殊功能的例子,以及这些功能的改变如何与癌症相关联。特别是,我们专注于:(i) 与不同适配器相关的货物选择和内吞机制;(ii) 网格蛋白介导与非网格蛋白内吞作用的特殊功能;(iii) 通过内吞蛋白的翻译后修饰对内吞机制的差异调节;(iv) 内吞蛋白的细胞环境依赖性表达和功能。作为恰当的例子,我们描述了两个内吞蛋白家族,动力蛋白和 epsins。最后,我们讨论了癌细胞如何利用这些特殊内吞蛋白的生理作用失调来增加细胞增殖、迁移和侵袭,从而导致抗凋亡或促转移行为。(iii) 通过内吞蛋白的翻译后修饰对内吞机制的差异调节;(iv) 内吞蛋白的细胞环境依赖性表达和功能。作为恰当的例子,我们描述了两个内吞蛋白家族,动力蛋白和 epsins。最后,我们讨论了癌细胞如何利用这些特殊内吞蛋白的生理作用失调来增加细胞增殖、迁移和侵袭,从而导致抗凋亡或促转移行为。(iii) 通过内吞蛋白的翻译后修饰对内吞机制的差异调节;(iv) 内吞蛋白的细胞环境依赖性表达和功能。作为恰当的例子,我们描述了两个内吞蛋白家族,动力蛋白和 epsins。最后,我们讨论了癌细胞如何利用这些特殊内吞蛋白的生理作用失调来增加细胞增殖、迁移和侵袭,从而导致抗凋亡或促转移行为。


























 京公网安备 11010802027423号
京公网安备 11010802027423号