当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibria for the n-Pentyl acetate, n-Hexyl acetate, n-Pentanol, or n-Hexanol + Furfural + Water Systems at 298 and 323 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-04 , DOI: 10.1021/acs.jced.0c00533 Marja Mikola 1 , Jani Kangas 1 , Juha-Pekka Pokki 2 , Mikael Männistö 2 , Juha Ahola 1 , Juha Tanskanen 1 , Ville Alopaeus 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-04 , DOI: 10.1021/acs.jced.0c00533 Marja Mikola 1 , Jani Kangas 1 , Juha-Pekka Pokki 2 , Mikael Männistö 2 , Juha Ahola 1 , Juha Tanskanen 1 , Ville Alopaeus 2
Affiliation
|
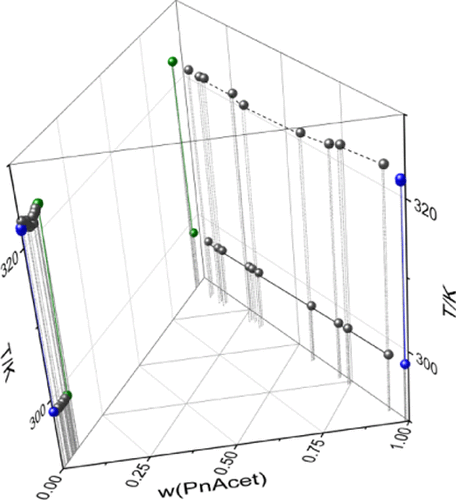
In this study, four ternary liquid–liquid equilibrium systems containing a solvent, furfural, and water were investigated by performing liquid–liquid equilibrium measurements at two different temperatures. The solvents were n-pentyl acetate, n-hexyl acetate, n-pentanol, and n-hexanol. The measurements were compared to the literature data and modeled with the UNIQUAC (universal quasi-chemical) activity coefficient model by taking into account also the vapor–liquid, liquid–liquid, and vapor–liquid–liquid equilibrium behaviors if present. n-Hexyl acetate showed the smallest solubility in water both in binary and ternary measurements. All four ternary systems showed similar magnitude distribution coefficients but furfural had the highest selectivity to n-hexyl acetate. Among the four systems investigated in this work, n-hexyl acetate was observed to have the best characteristics as a solvent for the extraction of furfural from water.
中文翻译:

液-液平衡Ñ戊基乙酸酯,Ñ己基乙酸酯,Ñ戊醇,或Ñ己醇+ +糠醛水系统在298和323K下
在这项研究中,通过在两个不同温度下进行液-液平衡测量,研究了包含溶剂,糠醛和水的四个三元液-液平衡系统。的溶剂是Ñ乙酸戊基,Ñ乙酸-己基,Ñ戊醇,和Ñ己醇。将测量值与文献数据进行比较,并通过考虑气-液,液-液和气-液-液平衡行为(如果存在),使用UNIQUAC(通用准化学)活度系数模型进行建模。ñ在二元和三元测量中,乙酸己酯在水中的溶解度最小。所有四个三元系统均显示出相似的幅度分布系数,但糠醛对乙酸正己酯的选择性最高。在这项工作研究的四个系统中,观察到乙酸正己酯作为从水中提取糠醛的溶剂具有最佳特性。
更新日期:2021-01-14
中文翻译:

液-液平衡Ñ戊基乙酸酯,Ñ己基乙酸酯,Ñ戊醇,或Ñ己醇+ +糠醛水系统在298和323K下
在这项研究中,通过在两个不同温度下进行液-液平衡测量,研究了包含溶剂,糠醛和水的四个三元液-液平衡系统。的溶剂是Ñ乙酸戊基,Ñ乙酸-己基,Ñ戊醇,和Ñ己醇。将测量值与文献数据进行比较,并通过考虑气-液,液-液和气-液-液平衡行为(如果存在),使用UNIQUAC(通用准化学)活度系数模型进行建模。ñ在二元和三元测量中,乙酸己酯在水中的溶解度最小。所有四个三元系统均显示出相似的幅度分布系数,但糠醛对乙酸正己酯的选择性最高。在这项工作研究的四个系统中,观察到乙酸正己酯作为从水中提取糠醛的溶剂具有最佳特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号