当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile Construction of an Amino-1,3-Oxazine Scaffold using Burgess Reagent Under Mild Conditions
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-12-05 , DOI: 10.1016/j.tetlet.2020.152684 Kouki Fuchino , Moriyasu Masui , Shuhei Yoshida , Ken-ichi Kusakabe
中文翻译:

在温和条件下使用Burgess试剂轻松构建1,3-Oxazine氨基支架
更新日期:2021-01-21
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-12-05 , DOI: 10.1016/j.tetlet.2020.152684 Kouki Fuchino , Moriyasu Masui , Shuhei Yoshida , Ken-ichi Kusakabe
|
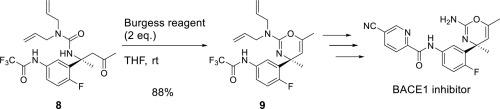
The development of a cyclization reaction to access amino-1,3-oxazines under mild conditions is described. The synthesis was achieved using dehydrating reagents, such as phosphorus pentoxide and Burgess reagent. In particular, the cyclization with Burgess reagent proceeded under mild conditions and tolerated potentially labile functional groups, such as the acetoxy group, and therefore can be used to synthesize β-secretase (BACE1) inhibitors with a variety of amino-1,3-oxazine warheads.
中文翻译:

在温和条件下使用Burgess试剂轻松构建1,3-Oxazine氨基支架
描述了在温和条件下获得氨基-1,3-恶嗪环化反应的进展。使用脱水剂如五氧化二磷和Burgess试剂可完成合成。特别是,使用Burgess试剂的环化反应在温和的条件下进行,并且可以耐受潜在不稳定的官能团(如乙酰氧基),因此可用于合成具有多种氨基-1,3-恶嗪的β-分泌酶(BACE1)抑制剂弹头。


























 京公网安备 11010802027423号
京公网安备 11010802027423号