当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theory of Electrochemical Proton-Coupled Electron Transfer in Diabatic Vibronic Representation: Application to Proton Discharge on Metal Electrodes in Alkaline Solution
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jpcc.0c08096 Yan-Choi Lam 1 , Alexander V. Soudackov 1 , Sharon Hammes-Schiffer 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jpcc.0c08096 Yan-Choi Lam 1 , Alexander V. Soudackov 1 , Sharon Hammes-Schiffer 1
Affiliation
|
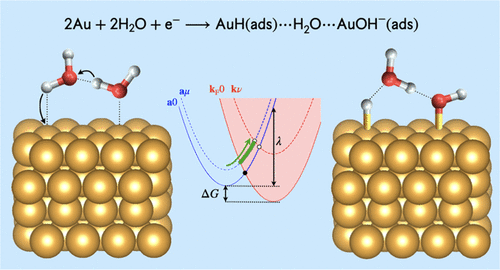
Proton discharge on a metal electrode is an important step in a wide range of electrocatalytic processes. In this proton-coupled electron transfer (PCET) reaction, also denoted the Volmer reaction, an acid in solution donates its proton to form a metal–hydrogen bond. Herein, a theoretical formulation is developed to describe this PCET reaction as a series of nonadiabatic transitions between reactant and product diabatic electron–proton vibronic states. The product states consist of overlapping continua of vibronic states associated with the continuum of delocalized electrode electronic states, leading to small coupling between each pair of vibronic states. The probability of reaching a crossing point between each pair is influenced by the probability of bypassing all other transitions. The resulting rate constant expression smoothly bridges the adiabatic and nonadiabatic limits for the entire range of electronic coupling and can be interpolated with the solvent-controlled regime. This theoretical formulation is applied to the Volmer reaction on a gold electrode in alkaline aqueous solution. The proposed mechanism involves proton discharge from a water dimer to the electrode surface simultaneously with proton transfer between the two water molecules and adsorption of hydroxide on the electrode. The calculated transfer coefficients and kinetic isotope effects are in qualitative agreement with experimental measurements, predicting a potential-dependent kinetic isotope effect. The decreasing kinetic isotope effect with less cathodic potentials is attributed to increasing contributions from excited reactant vibronic states, which are associated with larger and less isotope-dependent vibrational overlaps. This general theoretical formulation will enable the investigation of heterogeneous electrochemical PCET reactions spanning the adiabatic, nonadiabatic, and solvent-controlled regimes.
中文翻译:

绝热振动表示中质子耦合电子转移的理论:在碱性溶液中金属电极上的质子放电中的应用
在各种电催化过程中,金属电极上的质子放电是重要的一步。在这种质子耦合电子转移(PCET)反应(也称为Volmer反应)中,溶液中的酸将其质子赠与形成金属氢键。在这里,发展了一种理论公式来描述PCET反应是反应物和产物非绝热电子-质子振动态之间的一系列非绝热转变。乘积态包括与离域电极电子态连续体相关的重叠的振动态连续体,从而导致每对振动态之间的小耦合。绕过所有其他过渡的概率会影响到达每一对之间的交叉点的概率。所得的速率常数表达式可以平滑地桥接整个电子耦合范围的绝热和非绝热极限,并且可以用溶剂控制方案进行插值。该理论公式适用于碱性水溶液中金电极上的Volmer反应。所提出的机制涉及质子从水二聚体向电极表面的放电,同时质子在两个水分子之间转移以及氢氧化物在电极上的吸附。计算得出的转移系数和动力学同位素效应与实验测量结果在质量上吻合,从而预测了电位依赖性动力学同位素效应。阴极电位降低的动力学同位素效应降低归因于受激反应物振动态的贡献增加,与较大和较少的同位素相关的振动重叠相关。这种一般的理论表述将使人们能够研究跨越绝热,非绝热和溶剂控制方案的非均相电化学PCET反应。
更新日期:2020-12-17
中文翻译:

绝热振动表示中质子耦合电子转移的理论:在碱性溶液中金属电极上的质子放电中的应用
在各种电催化过程中,金属电极上的质子放电是重要的一步。在这种质子耦合电子转移(PCET)反应(也称为Volmer反应)中,溶液中的酸将其质子赠与形成金属氢键。在这里,发展了一种理论公式来描述PCET反应是反应物和产物非绝热电子-质子振动态之间的一系列非绝热转变。乘积态包括与离域电极电子态连续体相关的重叠的振动态连续体,从而导致每对振动态之间的小耦合。绕过所有其他过渡的概率会影响到达每一对之间的交叉点的概率。所得的速率常数表达式可以平滑地桥接整个电子耦合范围的绝热和非绝热极限,并且可以用溶剂控制方案进行插值。该理论公式适用于碱性水溶液中金电极上的Volmer反应。所提出的机制涉及质子从水二聚体向电极表面的放电,同时质子在两个水分子之间转移以及氢氧化物在电极上的吸附。计算得出的转移系数和动力学同位素效应与实验测量结果在质量上吻合,从而预测了电位依赖性动力学同位素效应。阴极电位降低的动力学同位素效应降低归因于受激反应物振动态的贡献增加,与较大和较少的同位素相关的振动重叠相关。这种一般的理论表述将使人们能够研究跨越绝热,非绝热和溶剂控制方案的非均相电化学PCET反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号