当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface Orientation and Pressure Dependence of CO2 Activation on Cu Surfaces
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jpcc.0c08262 Tian Yang 1, 2 , Tangjie Gu 2, 3 , Yong Han 3 , Weijia Wang 3 , Yi Yu 3 , Yijing Zang 1, 2 , Hui Zhang 1 , Baohua Mao 1 , Yimin Li 1, 3 , Bo Yang 3 , Zhi Liu 1, 3
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jpcc.0c08262 Tian Yang 1, 2 , Tangjie Gu 2, 3 , Yong Han 3 , Weijia Wang 3 , Yi Yu 3 , Yijing Zang 1, 2 , Hui Zhang 1 , Baohua Mao 1 , Yimin Li 1, 3 , Bo Yang 3 , Zhi Liu 1, 3
Affiliation
|
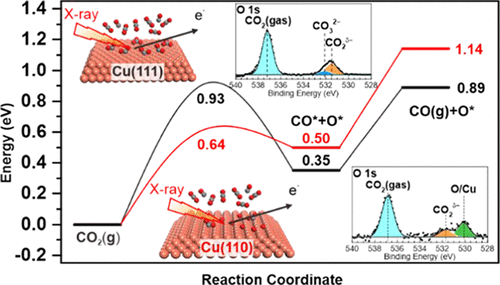
A fundamental understanding of interactions between catalysts and gas molecules is essential for the development of efficient heterogeneous catalysts. In this study, ambient pressure X-ray photoelectron spectroscopy (APXPS) and density functional theory (DFT) simulation were employed to investigate the activation of CO2 on Cu surfaces, which acts as a key step in the catalytic reduction of CO2. APXPS results show that CO2 is adsorbed as CO2δ− on the Cu(111) surface under a pressure of 0.01 mbar at 300 K. Adsorbed CO2δ− gets partially transformed into carbonate with an increase of pressure to 1 mbar due to the disproportionation reaction between CO2 molecules. Subsequent annealing of the Cu(111) surface in a CO2 atmosphere leads to the dissociation of CO2δ− and carbonate, and a transformation to a chemisorbed oxygen covered surface occurred at 400 K and elevated temperatures. However, on the Cu(110) surface, the CO2δ− gradually dissociates to CO and chemisorbed oxygen in the presence of 1 mbar of CO2 at room temperature. The self-deactivation of CO2 adsorption due to the atomic oxygen generated by CO2 dissociation is observed on both Cu(111) and Cu(110) surfaces. Moreover, these experimental results indicate that the Cu(110) surface is more active than the Cu(111) surface in breaking C–O bonds, which is consistent with the results of DFT simulations. Our findings indicate that the activation of CO2 on Cu surfaces is strongly surface orientation- and pressure-dependent, which is an important step to clarify CO2 activation mechanisms on Cu-based catalysts.
中文翻译:

铜表面上CO 2活化的表面取向和压力依赖性
对催化剂与气体分子之间相互作用的基本了解对于开发高效多相催化剂至关重要。在这项研究中,环境压力X射线光电子能谱法(APXPS)和密度泛函理论(DFT)模拟被雇用来调查CO的活化2在Cu表面,其作为在催化还原CO的一个关键步骤2。APXPS结果表明,CO 2被吸附的CO 2 δ- 0.01毫巴的在300K吸附CO的压力下在Cu(111)表面上的2 δ-被部分地与增加的压力转化为碳酸盐至1mbar由于CO 2之间的歧化反应分子。在CO在Cu(111)表面的后续退火2气氛导致CO的解离2 δ-和碳酸盐,和变换到化学吸附的氧气覆盖的表面发生在400度K和升高的温度。然而,在Cu(110)表面时,CO 2 δ-逐渐离解成CO和在1毫巴的CO的化学吸附存在氧气2在室温下。由于CO 2产生的原子氧,CO 2吸附的自失活在Cu(111)和Cu(110)表面均观察到解离。此外,这些实验结果表明,Cu(110)表面在打破C–O键方面比Cu(111)表面更具活性,这与DFT模拟的结果一致。我们的发现表明,Cu表面上的CO 2活化与表面取向和压力密切相关,这是阐明Cu基催化剂上CO 2活化机理的重要步骤。
更新日期:2020-12-17
中文翻译:

铜表面上CO 2活化的表面取向和压力依赖性
对催化剂与气体分子之间相互作用的基本了解对于开发高效多相催化剂至关重要。在这项研究中,环境压力X射线光电子能谱法(APXPS)和密度泛函理论(DFT)模拟被雇用来调查CO的活化2在Cu表面,其作为在催化还原CO的一个关键步骤2。APXPS结果表明,CO 2被吸附的CO 2 δ- 0.01毫巴的在300K吸附CO的压力下在Cu(111)表面上的2 δ-被部分地与增加的压力转化为碳酸盐至1mbar由于CO 2之间的歧化反应分子。在CO在Cu(111)表面的后续退火2气氛导致CO的解离2 δ-和碳酸盐,和变换到化学吸附的氧气覆盖的表面发生在400度K和升高的温度。然而,在Cu(110)表面时,CO 2 δ-逐渐离解成CO和在1毫巴的CO的化学吸附存在氧气2在室温下。由于CO 2产生的原子氧,CO 2吸附的自失活在Cu(111)和Cu(110)表面均观察到解离。此外,这些实验结果表明,Cu(110)表面在打破C–O键方面比Cu(111)表面更具活性,这与DFT模拟的结果一致。我们的发现表明,Cu表面上的CO 2活化与表面取向和压力密切相关,这是阐明Cu基催化剂上CO 2活化机理的重要步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号