当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multicomponent Solid–Liquid Equilibrium of 1,3,5-Triformylbenzene—A Key Intermediate for Porous Organic Cages: Solubility Determination and Correlation in Different Solvent Systems
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-04 , DOI: 10.1021/acs.jced.0c00776 Jingxuan Qiu 1 , Haishuang Huang 1 , Hui He 1 , Haoyou Liu 1, 2 , Shen Hu 1, 2 , Jiaming Han 1, 2 , Ying Guo 1, 2 , Peng Wang 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-04 , DOI: 10.1021/acs.jced.0c00776 Jingxuan Qiu 1 , Haishuang Huang 1 , Hui He 1 , Haoyou Liu 1, 2 , Shen Hu 1, 2 , Jiaming Han 1, 2 , Ying Guo 1, 2 , Peng Wang 1
Affiliation
|
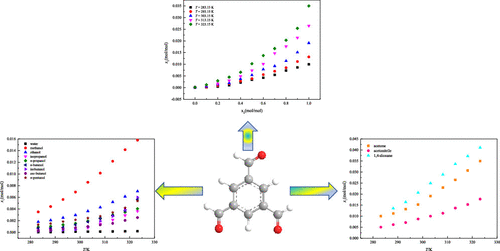
The multicomponent solid–liquid equilibrium data for 1,3,5-triformylbenzene, a key intermediate for the synthesis of porous organic cages, in 12 neat solvents (water, methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutanol, sec-butanol, n-pentanol, acetone, acetonitrile, and 1,4-dioxane) and the binary solvent mixture of acetone + water were measured by the static gravimetric method within the temperature range from 283.15 to 323.15 K. In both neat and binary solvent systems, the solubility values increased with the increase in temperature. Analysis of the data showed that the solubility behavior was mainly affected by the properties of solute and solvents. Six thermodynamic models, i.e., the modified Apelblat model, Yaws model, simplified Jouyban–Acree model, Machatha model, Apelblat–Jouyban–Acree model, and Apelblat–Machatha model, were used for correlating the obtained solubility data mathematically. The Yaws model could give a better fitting result for the solubility in pure solvents (the maximum ARD is 10.761 × 10–2), and the correlation result of Machatha model is the best for the binary solvent system (the largest ARD is 5.699 × 10–2). To evaluate the models for correlating the solubility data, the Akaike information criterion values and Akaike weights were calculated for each thermodynamic model.
中文翻译:

1,3,5-三甲酰基苯的多组分固液平衡-多孔有机笼的关键中间体:不同溶剂体系中的溶解度测定和相关性
在12种纯溶剂(水,甲醇,乙醇,正丙醇,异丙醇,正丁醇,异丁醇,仲丁醇n-戊醇,丙酮,乙腈和1,4-二恶烷)和丙酮+水的二元溶剂混合物通过静态重量法在283.15至323.15 K的温度范围内进行测量。在纯净和二元溶剂系统中,溶解度值随温度的升高而增加。数据分析表明,溶解度行为主要受溶质和溶剂性质的影响。使用六个热力学模型,即改良的Apelblat模型,Yaws模型,简化的Jouyban-Acree模型,Machatha模型,Apelblat-Jouyban-Acree模型和Apelblat-Machatha模型,对获得的溶解度数据进行数学关联。Yaws模型可以为纯溶剂中的溶解度提供更好的拟合结果(最大ARD为10.761×10 –2),Machatha模型的相关结果对于二元溶剂系统来说是最好的(最大ARD为5.699×10 –2)。为了评估用于关联溶解度数据的模型,针对每个热力学模型计算了Akaike信息标准值和Akaike权重。
更新日期:2021-01-14
中文翻译:

1,3,5-三甲酰基苯的多组分固液平衡-多孔有机笼的关键中间体:不同溶剂体系中的溶解度测定和相关性
在12种纯溶剂(水,甲醇,乙醇,正丙醇,异丙醇,正丁醇,异丁醇,仲丁醇n-戊醇,丙酮,乙腈和1,4-二恶烷)和丙酮+水的二元溶剂混合物通过静态重量法在283.15至323.15 K的温度范围内进行测量。在纯净和二元溶剂系统中,溶解度值随温度的升高而增加。数据分析表明,溶解度行为主要受溶质和溶剂性质的影响。使用六个热力学模型,即改良的Apelblat模型,Yaws模型,简化的Jouyban-Acree模型,Machatha模型,Apelblat-Jouyban-Acree模型和Apelblat-Machatha模型,对获得的溶解度数据进行数学关联。Yaws模型可以为纯溶剂中的溶解度提供更好的拟合结果(最大ARD为10.761×10 –2),Machatha模型的相关结果对于二元溶剂系统来说是最好的(最大ARD为5.699×10 –2)。为了评估用于关联溶解度数据的模型,针对每个热力学模型计算了Akaike信息标准值和Akaike权重。



























 京公网安备 11010802027423号
京公网安备 11010802027423号