当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The crystal structure of benzophenone synthase from Garcinia mangostana L. pericarps reveals the basis for substrate specificity and catalysis
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-12-04 , DOI: 10.1107/s2053230x20014818 Chomphunuch Songsiriritthigul , Natsajee Nualkaew , James Ketudat-Cairns , Chun-Jung Chen
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-12-04 , DOI: 10.1107/s2053230x20014818 Chomphunuch Songsiriritthigul , Natsajee Nualkaew , James Ketudat-Cairns , Chun-Jung Chen
|
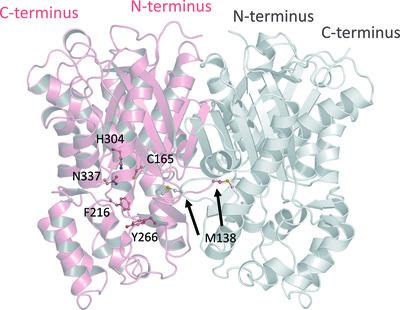
Benzophenone synthase (BPS) catalyzes the production of 2,4,6‐trihydroxybenzophenone via the condensation of benzoyl‐CoA and three units of malonyl‐CoA. The biosynthetic pathway proceeds with the formation of the prenylated xanthone α‐mangostin from 2,4,6‐trihydroxybenzophenone. Structural elucidation was performed to gain a better understanding of the structural basis of the function of Garcinia mangostana L. (mangosteen) BPS (GmBPS). The structure reveals the common core consisting of a five‐layer αβαβα fold as found in other type III polyketide synthase enzymes. The three residues Met264, Tyr266 and Gly339 are proposed to have a significant impact on the substrate‐binding specificity of the active site. Crystallographic and docking studies indicate why benzoyl‐CoA is preferred over 4‐coumaroyl‐CoA as the substrate for GmBPS. Met264 and Tyr266 in GmBPS are properly oriented for accommodation of the 2,4,6‐trihydroxybenzophenone product but not of naringenin. Gly339 offers a minimal steric hindrance to accommodate the extended substrate. Moreover, the structural arrangement of Thr133 provides the elongation activity and consequently facilitates extension of the polyketide chain. In addition to its impact on the substrate selectivity, Ala257 expands the horizontal cavity and might serve to facilitate the initiation/cyclization reaction. The detailed structure of GmBPS explains its catalytic function, facilitating further structure‐based engineering to alter its substrate specificity and obtain the desired products.
中文翻译:

Garcinia mangostana L. pericarps 二苯甲酮合酶的晶体结构揭示了底物特异性和催化的基础
二苯甲酮合酶(BPS)通过苯甲酰辅酶A和三个丙二酰辅酶A单元的缩合催化2,4,6-三羟基二苯甲酮的产生。生物合成途径是从 2,4,6-三羟基二苯甲酮形成异戊二烯化氧杂蒽酮 α-芒果素。进行结构解析以更好地了解藤黄果功能的结构基础L. (山竹) BPS (GmBPS)。该结构揭示了在其他 III 型聚酮化合物合酶中发现的由五层 αβαβα 折叠组成的共同核心。提出三个残基 Met264、Tyr266 和 Gly339 对活性位点的底物结合特异性有显着影响。晶体学和对接研究表明,为什么苯甲酰辅酶 A 优于 4-香豆酰辅酶 A 作为 GmBPS 的底物。GmBPS 中的 Met264 和 Tyr266 被正确定向以适应 2,4,6-三羟基二苯甲酮产物而不是柚皮素。Gly339 提供最小的空间位阻以容纳扩展的底物。此外,Thr133 的结构排列提供了延伸活性,从而促进了聚酮链的延伸。除了对底物选择性的影响外,Ala257 扩大了水平腔,可能有助于引发/环化反应。GmBPS 的详细结构解释了其催化功能,有助于进一步基于结构的工程改变其底物特异性并获得所需的产物。
更新日期:2020-12-04
中文翻译:

Garcinia mangostana L. pericarps 二苯甲酮合酶的晶体结构揭示了底物特异性和催化的基础
二苯甲酮合酶(BPS)通过苯甲酰辅酶A和三个丙二酰辅酶A单元的缩合催化2,4,6-三羟基二苯甲酮的产生。生物合成途径是从 2,4,6-三羟基二苯甲酮形成异戊二烯化氧杂蒽酮 α-芒果素。进行结构解析以更好地了解藤黄果功能的结构基础L. (山竹) BPS (GmBPS)。该结构揭示了在其他 III 型聚酮化合物合酶中发现的由五层 αβαβα 折叠组成的共同核心。提出三个残基 Met264、Tyr266 和 Gly339 对活性位点的底物结合特异性有显着影响。晶体学和对接研究表明,为什么苯甲酰辅酶 A 优于 4-香豆酰辅酶 A 作为 GmBPS 的底物。GmBPS 中的 Met264 和 Tyr266 被正确定向以适应 2,4,6-三羟基二苯甲酮产物而不是柚皮素。Gly339 提供最小的空间位阻以容纳扩展的底物。此外,Thr133 的结构排列提供了延伸活性,从而促进了聚酮链的延伸。除了对底物选择性的影响外,Ala257 扩大了水平腔,可能有助于引发/环化反应。GmBPS 的详细结构解释了其催化功能,有助于进一步基于结构的工程改变其底物特异性并获得所需的产物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号