Journal of Rare Earths ( IF 4.9 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.jre.2020.11.021 Bin Ji , Qi Li , Wencai Zhang
|
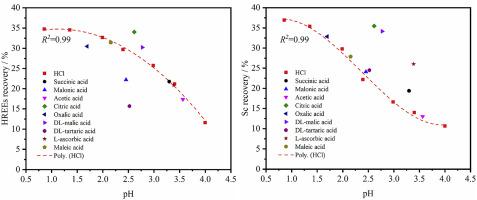
Due to the increasing criticality of rare earth elements (REEs), it has become essential to recover REEs from alternative resources. In this study, systematic REEs leaching tests were performed on the calcination product of a coal coarse refuse using hydrochloric acid and different types of organic acid as lixiviants. Experimental results show that the recovery of REEs, especially heavy REEs (HREEs) and scandium (Sc), is improved by using selected organic acids. Citric acid and dl-malic acid afford the best leaching performances; whereas, malonic acid, oxalic acid, and dl-tartaric acid are inferior to hydrochloric acid. Results of zeta potential measurements and solution chemical equilibrium calculations show that malonic acid is more likely adsorbed on the surface of the calcined material compared with citric acid and dl-malic acid. The adsorption may reduce the effective concentration of malonic species in solution and/or increase the amount of REEs adsorbed on the surface, thereby impairing the leaching recovery. Compared with light REEs (LREEs), a stronger adsorption of the HREEs on the surface is observed from electro-kinetic test results. This finding explains why organic acids impose a more positive impact on the leaching recovery of HREEs. By complexing with the HREEs, organic acids can keep the metal ions in solution and improve the leaching recovery. The adsorption of Sc3+ on the surface is the lowest compared with other REEs. Therefore, rather than complexing, the organic anionic species likely play a function of solubilizing Sc from the solid, which is similar to that of hydrogen ions.
中文翻译:

有机酸从煤粗矸石煅烧产物中浸出回收稀土元素
由于稀土元素 (REE) 的重要性日益增加,从替代资源中回收 REE 变得至关重要。本研究以盐酸和不同类型的有机酸为浸出剂,对煤粗矸石的煅烧产物进行了系统的稀土元素浸出试验。实验结果表明,通过选择有机酸可以提高稀土元素的回收率,尤其是重稀土元素(HREEs)和钪(Sc)。柠檬酸和dl-苹果酸的浸出性能最好;而丙二酸、草酸和dl-酒石酸不如盐酸。zeta 电位测量和溶液化学平衡计算结果表明,与柠檬酸和dl相比,丙二酸更容易吸附在煅烧材料的表面。-苹果酸。吸附可能会降低溶液中丙二酸物质的有效浓度和/或增加吸附在表面上的 REE 的量,从而削弱浸出回收率。与轻稀土元素(LREEs)相比,从电动测试结果中可以观察到高稀土元素在表面的吸附更强。这一发现解释了为什么有机酸对高稀土元素的浸出回收率产生更积极的影响。通过与高稀土元素的络合,有机酸可以使金属离子保持在溶液中,提高浸出回收率。与其他稀土元素相比,Sc 3+在表面的吸附是最低的。因此,有机阴离子物种可能发挥从固体中溶解 Sc 的功能,而不是络合,这类似于氢离子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号