Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.bbamem.2020.183529 Pontus Pettersson , Joan Patrick , Mario Jakob , Malte Jacobs , Ralf Bernd Klösgen , Stefan Wennmalm , Lena Mäler
|
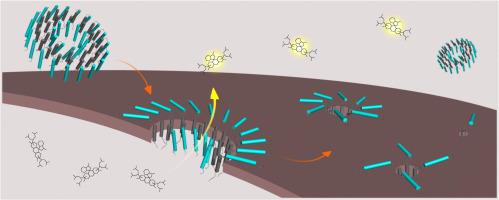
The twin-arginine translocase (Tat) mediates the transport of already-folded proteins across membranes in bacteria, plants and archaea. TatA is a small, dynamic subunit of the Tat-system that is believed to be the active component during target protein translocation. TatA is foremost characterized as a bitopic membrane protein, but has also been found to partition into a soluble, oligomeric structure of yet unknown function. To elucidate the interplay between the membrane-bound and soluble forms we have investigated the oligomers formed by Arabidopsis thaliana TatA. We used several biophysical techniques to study the oligomeric structure in solution, the conversion that takes place upon interaction with membrane models of different compositions, and the effect on bilayer integrity upon insertion. Our results demonstrate that in solution TatA oligomerizes into large objects with a high degree of ordered structure. Upon interaction with lipids, conformational changes take place and TatA disintegrates into lower order oligomers. The insertion of TatA into lipid bilayers causes a temporary leakage of small molecules across the bilayer. The disruptive effect on the membrane is dependent on the liposome's negative surface charge density, with more leakage observed for purely zwitterionic bilayers. Overall, our findings indicate that A. thaliana TatA forms oligomers in solution that insert into bilayers, a process that involves reorganization of the protein oligomer.
中文翻译:

可溶性TatA形成与膜相互作用的寡聚物:多功能蛋白转运蛋白的结构和插入研究
双精氨酸转位酶(Tat)介导细菌,植物和古细菌中已经折叠的蛋白质跨膜运输。TatA是Tat系统的一个小的动态亚基,被认为是靶蛋白易位期间的活性成分。TatA最主要的特征是双位膜蛋白,但也发现它可以分成功能未知的可溶性寡聚结构。为了阐明膜结合形式和可溶性形式之间的相互作用,我们研究了拟南芥形成的寡聚物塔塔 我们使用了几种生物物理技术来研究溶液中的低聚结构,与不同组成的膜模型相互作用时发生的转化以及插入时对双层完整性的影响。我们的结果表明,在溶液中TatA会低聚为具有高度有序结构的大物体。与脂质相互作用后,构象发生变化,TatA分解为低阶寡聚物。TatA插入脂质双层中导致小分子暂时泄漏穿过双层。对膜的破坏作用取决于脂质体的负表面电荷密度,对于纯两性离子双层膜观察到更多的泄漏。总体而言,我们的发现表明拟南芥 TatA在插入双层中的溶液中形成寡聚物,该过程涉及蛋白质寡聚物的重组。


























 京公网安备 11010802027423号
京公网安备 11010802027423号