当前位置:
X-MOL 学术
›
Int. J. Energy Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solution‐phase‐reconstructed Zn‐based nanowire electrocatalysts for electrochemical reduction of carbon dioxide to carbon monoxide
International Journal of Energy Research ( IF 4.6 ) Pub Date : 2020-12-02 , DOI: 10.1002/er.6276 Junhyeong Kim 1 , Hyunki Kim 1 , Gyeong Ho Han 1 , Sang Hyun Ahn 1
International Journal of Energy Research ( IF 4.6 ) Pub Date : 2020-12-02 , DOI: 10.1002/er.6276 Junhyeong Kim 1 , Hyunki Kim 1 , Gyeong Ho Han 1 , Sang Hyun Ahn 1
Affiliation
|
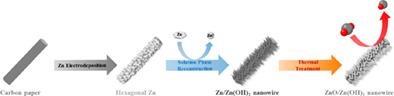
The production of CO via electrochemical CO2 reduction has been recognised as a promising technology that overcomes the environmental issues caused by global warming. It also facilitates the conversion of CO2 into energy sources. Earth‐abundant Zn is a well‐known alternative to noble metal catalysts such as Au and Ag for the electrochemical CO2 reduction to CO. In particular, Zn‐based materials in the form of nanowires are potentially applicable as electrocatalysts in the electrochemical CO2 reduction. However, the conventional methods for manufacturing the nanowire structure are difficult as they require harsh conditions such as high temperatures and excess energy. In this study, Zn‐based nanowire catalysts are prepared by the facile electrodeposition of Zn nanostructures on a substrate followed by their energy‐free solution‐phase reconstitution. Further, their electrochemical performance in the CO2 reduction is investigated in a CO2‐purged 0.5 M KHCO3 electrolyte. By optimising the deposition conditions, hexagonal Zn (h‐Zn) plates with dominant Zn(101) facets, the favoured crystal structure for CO2 reduction, are fabricated on carbon paper. Furthermore, it is found that, during the solution‐phase reconstruction over 16 hours, the h‐Zn plate transforms to a nanowire owing to the differences between the oxidation rates of different crystal facets and the formation of a hydroxide complex. The activity of the reconstructed Zn‐based nanowire catalyst is enhanced further by forming an oxide layer via thermal treatment in a H2 atmosphere. This treatment boosted the reaction kinetics, thereby enhancing the catalyst performance in the CO2 reduction.
中文翻译:

固溶相重构的锌基纳米线电催化剂,用于将二氧化碳电化学还原为一氧化碳
通过电化学还原CO 2来生产CO已经被认为是一种有前途的技术,它克服了由全球变暖引起的环境问题。它还有利于将CO 2转化为能源。富含地球的锌是Au和Ag等贵金属催化剂的公知替代品,用于将CO 2电化学还原为CO。特别是,纳米线形式的Zn基材料有可能用作电化学CO 2中的电催化剂。减少。然而,用于制造纳米线结构的常规方法是困难的,因为它们需要苛刻的条件,例如高温和多余的能量。在这项研究中,基于Zn的纳米线催化剂是通过在衬底上轻松电沉积Zn纳米结构,然后进行无能量的溶液相重构而制备的。此外,在CO 2净化的0.5 M KHCO 3电解质中研究了它们在CO 2还原中的电化学性能。通过优化沉积条件,具有占优势的Zn(101)面的六角形Zn(h-Zn)板,成为CO 2的首选晶体结构还原,是在复写纸上制作的。此外,发现在16小时以上的固溶相重构过程中,由于不同晶面的氧化速率与氢氧化物络合物形成之间的差异,h-Zn板转变为纳米线。通过在H 2气氛中进行热处理形成氧化层,可以进一步增强重建的Zn基纳米线催化剂的活性。该处理提高了反应动力学,从而提高了CO 2还原中的催化剂性能。
更新日期:2020-12-02
中文翻译:

固溶相重构的锌基纳米线电催化剂,用于将二氧化碳电化学还原为一氧化碳
通过电化学还原CO 2来生产CO已经被认为是一种有前途的技术,它克服了由全球变暖引起的环境问题。它还有利于将CO 2转化为能源。富含地球的锌是Au和Ag等贵金属催化剂的公知替代品,用于将CO 2电化学还原为CO。特别是,纳米线形式的Zn基材料有可能用作电化学CO 2中的电催化剂。减少。然而,用于制造纳米线结构的常规方法是困难的,因为它们需要苛刻的条件,例如高温和多余的能量。在这项研究中,基于Zn的纳米线催化剂是通过在衬底上轻松电沉积Zn纳米结构,然后进行无能量的溶液相重构而制备的。此外,在CO 2净化的0.5 M KHCO 3电解质中研究了它们在CO 2还原中的电化学性能。通过优化沉积条件,具有占优势的Zn(101)面的六角形Zn(h-Zn)板,成为CO 2的首选晶体结构还原,是在复写纸上制作的。此外,发现在16小时以上的固溶相重构过程中,由于不同晶面的氧化速率与氢氧化物络合物形成之间的差异,h-Zn板转变为纳米线。通过在H 2气氛中进行热处理形成氧化层,可以进一步增强重建的Zn基纳米线催化剂的活性。该处理提高了反应动力学,从而提高了CO 2还原中的催化剂性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号