当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Density, Viscosity, and Electrical Conductivity of 1-Alkyl-3-methylimidazolium Dicyanamide Ionic Liquids
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.jced.0c00754 Yong Zheng 1 , Yongjun Zheng 1 , Qian Wang 2 , Zhen Wang 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.jced.0c00754 Yong Zheng 1 , Yongjun Zheng 1 , Qian Wang 2 , Zhen Wang 1
Affiliation
|
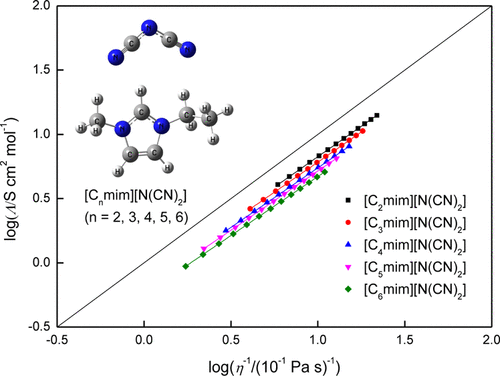
A series of 1-alkyl-3-methylimidazolium dicyanamide ionic liquids ([Cnmim][N(CN)2], n = 2, 3, 4, 5, and 6) were synthesized and characterized in this work. The density, viscosity, and electrical conductivity of these ionic liquids were measured from 293.15 to 353.15 K at 101.3 kPa using a high-precision vibrating tube density meter, an automated falling ball viscometer, and a conductivity meter, respectively. On this basis, the corresponding molar volume and molar conductivity were also obtained. All experimental values were well-fitted by the empirical equations. Remarkably, these ionic liquids exhibit much lower density, lower viscosity, and higher electrical conductivity than many other conventional ionic liquids. According to the theoretical calculations, the weaker cation–anion interaction and V-shaped and nonspherical structure of the dicyanamide anion are considered as major reasons for these properties. Meanwhile, the extension of the alkyl side chain length can result in lower density, higher molar volume, higher viscosity, and lower conductivity. This phenomenon is probably ascribed to the decreasing molecular symmetry, enhanced hydrogen bonding, and increasing steric hindrance of ionic liquids. The relationship between viscosity and molar conductivity has been also investigated using the Walden rule, which confirms the high ionicity of [Cnmim][N(CN)2]. The enhanced dissociation of ions may favor the ionic fluidity and conductivity. It is hoped that these experimental results and theoretical findings may be useful for the future development of dicyanamide-based ionic liquids.
中文翻译:

1-烷基-3-甲基咪唑鎓双氰胺离子液体的密度,粘度和电导率
一系列1-烷基-3-甲基咪唑鎓双氰胺离子液体([C n mim] [N(CN)2 ],n= 2、3、4、5和6),并在这项工作中进行了表征。这些离子液体的密度,粘度和电导率分别使用高精度振动管密度仪,自动落球粘度计和电导仪在101.3 kPa下从293.15至353.15 K测量。在此基础上,也获得了相应的摩尔体积和摩尔电导率。所有实验值均通过经验方程式很好地拟合。值得注意的是,这些离子液体比许多其他常规离子液体表现出低得多的密度,更低的粘度和更高的电导率。根据理论计算,认为阳离子与阴离子之间的相互作用较弱,以及双氰胺阴离子的V形和非球形结构是造成这些性质的主要原因。与此同时,烷基侧链长度的延长可导致较低的密度,较高的摩尔体积,较高的粘度和较低的电导率。此现象可能归因于分子对称性的降低,氢键的增强以及离子液体的位阻增加。还使用瓦尔登法则研究了粘度与摩尔电导率之间的关系,该法则证实了[Cn mim] [N(CN) 2 ]。离子解离的增强可能有利于离子流动性和电导率。希望这些实验结果和理论发现可能对将来基于双氰胺的离子液体的开发有用。
更新日期:2021-01-14
中文翻译:

1-烷基-3-甲基咪唑鎓双氰胺离子液体的密度,粘度和电导率
一系列1-烷基-3-甲基咪唑鎓双氰胺离子液体([C n mim] [N(CN)2 ],n= 2、3、4、5和6),并在这项工作中进行了表征。这些离子液体的密度,粘度和电导率分别使用高精度振动管密度仪,自动落球粘度计和电导仪在101.3 kPa下从293.15至353.15 K测量。在此基础上,也获得了相应的摩尔体积和摩尔电导率。所有实验值均通过经验方程式很好地拟合。值得注意的是,这些离子液体比许多其他常规离子液体表现出低得多的密度,更低的粘度和更高的电导率。根据理论计算,认为阳离子与阴离子之间的相互作用较弱,以及双氰胺阴离子的V形和非球形结构是造成这些性质的主要原因。与此同时,烷基侧链长度的延长可导致较低的密度,较高的摩尔体积,较高的粘度和较低的电导率。此现象可能归因于分子对称性的降低,氢键的增强以及离子液体的位阻增加。还使用瓦尔登法则研究了粘度与摩尔电导率之间的关系,该法则证实了[Cn mim] [N(CN) 2 ]。离子解离的增强可能有利于离子流动性和电导率。希望这些实验结果和理论发现可能对将来基于双氰胺的离子液体的开发有用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号