当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
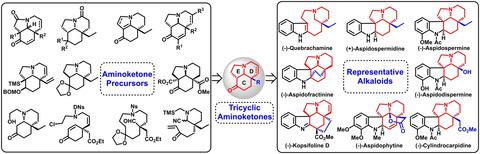
Synthetic Approaches to Tricyclic Aminoketones in the Total Synthesis of Aspidosperma and Kopsia Alkaloids
The Chemical Record ( IF 6.6 ) Pub Date : 2020-12-02 , DOI: 10.1002/tcr.202000131 Nengzhong Wang 1 , Xuefeng Jiang 1, 2
The Chemical Record ( IF 6.6 ) Pub Date : 2020-12-02 , DOI: 10.1002/tcr.202000131 Nengzhong Wang 1 , Xuefeng Jiang 1, 2
Affiliation
|
Aspidosperma and kopsia alkaloids are significant functional molecules because of their potent biological activities. Their intricate structures present an intrinsic synthetic challenge and thus attract significant attention from synthetic organic academic community. Over the past decades, a series of elegant strategies has been developed, in particular, the Stork's original Fischer indolization of tricyclic aminoketones 1. Herein, we report a comprehensive review on various synthetic approaches access to tricyclic aminoketones 1 and provide a practical guidance to readers whose are interested in employing tricyclic aminoketones 1 as versatile building blocks in the realm of total synthesis of aspidosperma, kopsia and structurally related alkaloids.
中文翻译:

曲霉和小菜碱生物碱的全合成中三环胺酮的合成方法
由于其有效的生物学活性,因此,精子植物和香菜碱生物碱是重要的功能分子。它们复杂的结构提出了内在的合成挑战,因此引起了合成有机学术界的极大关注。在过去的几十年中,已经开发出了一系列优雅的策略,特别是Stork最初对三环氨基酮进行的Fischer吲哚化1。在此,我们报告了有关获取三环氨基酮1的各种合成方法的全面综述,并为有兴趣将三环氨基酮1用作曲霉精子全合成领域的通用构建模块的读者提供了实用指南。,kopsia和与结构相关的生物碱。
更新日期:2020-12-02
中文翻译:

曲霉和小菜碱生物碱的全合成中三环胺酮的合成方法
由于其有效的生物学活性,因此,精子植物和香菜碱生物碱是重要的功能分子。它们复杂的结构提出了内在的合成挑战,因此引起了合成有机学术界的极大关注。在过去的几十年中,已经开发出了一系列优雅的策略,特别是Stork最初对三环氨基酮进行的Fischer吲哚化1。在此,我们报告了有关获取三环氨基酮1的各种合成方法的全面综述,并为有兴趣将三环氨基酮1用作曲霉精子全合成领域的通用构建模块的读者提供了实用指南。,kopsia和与结构相关的生物碱。


























 京公网安备 11010802027423号
京公网安备 11010802027423号