当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of two subtilisin‐like serine proteases engaged in the degradation of recombinant proteins in Nicotiana benthamiana
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-11 , DOI: 10.1002/1873-3468.14014 Alejandro A Puchol Tarazona 1 , Daniel Maresch 2 , Annette Grill 1 , Janet Bakalarz 1 , Juan A Torres Acosta 1 , Alexandra Castilho 1 , Herta Steinkellner 1 , Lukas Mach 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-11 , DOI: 10.1002/1873-3468.14014 Alejandro A Puchol Tarazona 1 , Daniel Maresch 2 , Annette Grill 1 , Janet Bakalarz 1 , Juan A Torres Acosta 1 , Alexandra Castilho 1 , Herta Steinkellner 1 , Lukas Mach 1
Affiliation
|
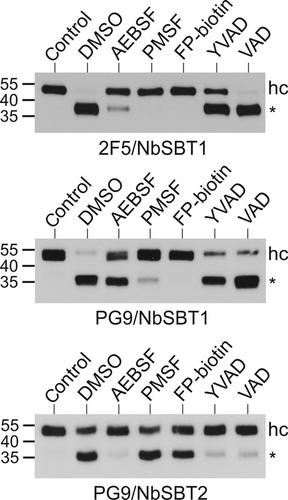
The tobacco variant Nicotiana benthamiana has recently emerged as a versatile host for the manufacturing of protein therapeutics, but the fidelity of many recombinant proteins generated in this system is compromised by inadvertent proteolysis. Previous studies have revealed that the anti-HIV-1 antibodies 2F5 and PG9 as well as the protease inhibitor α1 -antitrypsin are particularly susceptible to N. benthamiana proteases. Here we identify two subtilisin-like serine proteases (NbSBT1 and NbSBT2) whose combined action is sufficient to account for all major cleavage events observed upon expression of 2F5, PG9 and α1 -antitrypsin in N. benthamiana. We propose that down-regulation of NbSBT1 and NbSBT2 activities could constitute a powerful means to optimize the performance of this promising platform for the production of biopharmaceuticals.
中文翻译:

两种参与降解本氏烟草重组蛋白的枯草杆菌蛋白酶样丝氨酸蛋白酶的鉴定
烟草变种本氏烟草最近已成为制造蛋白质治疗剂的多功能宿主,但在该系统中生成的许多重组蛋白质的保真度因无意的蛋白水解而受到损害。先前的研究表明,抗 HIV-1 抗体 2F5 和 PG9 以及蛋白酶抑制剂 α1 -抗胰蛋白酶对本氏烟草蛋白酶特别敏感。在这里,我们鉴定了两种枯草杆菌蛋白酶样丝氨酸蛋白酶(NbSBT1 和 NbSBT2),它们的组合作用足以解释在本氏烟草中 2F5、PG9 和 α1-抗胰蛋白酶表达时观察到的所有主要切割事件。我们建议下调 NbSBT1 和 NbSBT2 活性可能是优化这一有前景的生物制药生产平台性能的有力手段。
更新日期:2020-12-11
中文翻译:

两种参与降解本氏烟草重组蛋白的枯草杆菌蛋白酶样丝氨酸蛋白酶的鉴定
烟草变种本氏烟草最近已成为制造蛋白质治疗剂的多功能宿主,但在该系统中生成的许多重组蛋白质的保真度因无意的蛋白水解而受到损害。先前的研究表明,抗 HIV-1 抗体 2F5 和 PG9 以及蛋白酶抑制剂 α1 -抗胰蛋白酶对本氏烟草蛋白酶特别敏感。在这里,我们鉴定了两种枯草杆菌蛋白酶样丝氨酸蛋白酶(NbSBT1 和 NbSBT2),它们的组合作用足以解释在本氏烟草中 2F5、PG9 和 α1-抗胰蛋白酶表达时观察到的所有主要切割事件。我们建议下调 NbSBT1 和 NbSBT2 活性可能是优化这一有前景的生物制药生产平台性能的有力手段。


























 京公网安备 11010802027423号
京公网安备 11010802027423号