当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystallographic binding studies of rat peroxisomal multifunctional enzyme type 1 with 3‐ketodecanoyl‐CoA: capturing active and inactive states of its hydratase and dehydrogenase catalytic sites
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2020-12-02 , DOI: 10.1107/s2059798320013819 Shruthi Sridhar 1 , Werner Schmitz 2 , J Kalervo Hiltunen 1 , Rajaram Venkatesan 1 , Ulrich Bergmann 3 , Tiila Riikka Kiema 3 , Rikkert K Wierenga 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2020-12-02 , DOI: 10.1107/s2059798320013819 Shruthi Sridhar 1 , Werner Schmitz 2 , J Kalervo Hiltunen 1 , Rajaram Venkatesan 1 , Ulrich Bergmann 3 , Tiila Riikka Kiema 3 , Rikkert K Wierenga 1
Affiliation
|
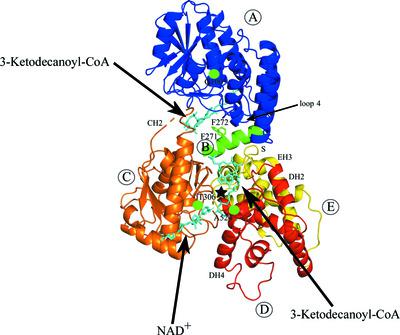
The peroxisomal multifunctional enzyme type 1 (MFE1) catalyzes two successive reactions in the β‐oxidation cycle: the 2E‐enoyl‐CoA hydratase (ECH) and NAD+‐dependent 3S‐hydroxyacyl‐CoA dehydrogenase (HAD) reactions. MFE1 is a monomeric enzyme that has five domains. The N‐terminal part (domains A and B) adopts the crotonase fold and the C‐terminal part (domains C, D and E) adopts the HAD fold. A new crystal form of MFE1 has captured a conformation in which both active sites are noncompetent. This structure, at 1.7 Å resolution, shows the importance of the interactions between Phe272 in domain B (the linker helix; helix H10 of the crotonase fold) and the beginning of loop 2 (of the crotonase fold) in stabilizing the competent ECH active‐site geometry. In addition, protein crystallographic binding studies using optimized crystal‐treatment protocols have captured a structure with both the 3‐ketodecanoyl‐CoA product and NAD+ bound in the HAD active site, showing the interactions between 3‐ketodecanoyl‐CoA and residues of the C, D and E domains. Structural comparisons show the importance of domain movements, in particular of the C domain with respect to the D/E domains and of the A domain with respect to the HAD part. These comparisons suggest that the N‐terminal part of the linker helix, which interacts tightly with domains A and E, functions as a hinge region for movement of the A domain with respect to the HAD part.
中文翻译:

大鼠过氧化物酶体多功能酶 1 型与 3-酮癸酰辅酶 A 的晶体结合研究:捕获其水合酶和脱氢酶催化位点的活性和非活性状态
过氧化物酶体多功能酶 1 (MFE1) 催化 β-氧化循环中的两个连续反应:2 E-烯酰-CoA 水合酶 (ECH) 和 NAD +依赖的 3 S-羟酰基辅酶A脱氢酶(HAD)反应。MFE1 是一种单体酶,具有五个结构域。N 端部分(域 A 和 B)采用巴豆酶折叠,C 端部分(域 C、D 和 E)采用 HAD 折叠。一种新的 MFE1 晶体形式已经捕获了一种构象,其中两个活性位点都没有能力。该结构以 1.7 Å 分辨率显示了结构域 B(接头螺旋;巴豆酶折叠的螺旋 H10)和环 2 开始(巴豆酶折叠)之间的相互作用在稳定有效 ECH 活性方面的重要性。场地几何。此外,使用优化的晶体处理方案的蛋白质晶体结合研究已经捕获了具有 3-酮癸酰辅酶 A 产物和 NAD + 的结构。结合在 HAD 活性位点,显示了 3-酮癸酰辅酶 A 与 C、D 和 E 结构域残基之间的相互作用。结构比较显示了域移动的重要性,尤其是 C 域相对于 D/E 域和 A 域相对于 HAD 部分的重要性。这些比较表明,接头螺旋的 N 端部分与结构域 A 和 E 紧密相互作用,作为 A 结构域相对于 HAD 部分运动的铰链区。
更新日期:2020-12-02
中文翻译:

大鼠过氧化物酶体多功能酶 1 型与 3-酮癸酰辅酶 A 的晶体结合研究:捕获其水合酶和脱氢酶催化位点的活性和非活性状态
过氧化物酶体多功能酶 1 (MFE1) 催化 β-氧化循环中的两个连续反应:2 E-烯酰-CoA 水合酶 (ECH) 和 NAD +依赖的 3 S-羟酰基辅酶A脱氢酶(HAD)反应。MFE1 是一种单体酶,具有五个结构域。N 端部分(域 A 和 B)采用巴豆酶折叠,C 端部分(域 C、D 和 E)采用 HAD 折叠。一种新的 MFE1 晶体形式已经捕获了一种构象,其中两个活性位点都没有能力。该结构以 1.7 Å 分辨率显示了结构域 B(接头螺旋;巴豆酶折叠的螺旋 H10)和环 2 开始(巴豆酶折叠)之间的相互作用在稳定有效 ECH 活性方面的重要性。场地几何。此外,使用优化的晶体处理方案的蛋白质晶体结合研究已经捕获了具有 3-酮癸酰辅酶 A 产物和 NAD + 的结构。结合在 HAD 活性位点,显示了 3-酮癸酰辅酶 A 与 C、D 和 E 结构域残基之间的相互作用。结构比较显示了域移动的重要性,尤其是 C 域相对于 D/E 域和 A 域相对于 HAD 部分的重要性。这些比较表明,接头螺旋的 N 端部分与结构域 A 和 E 紧密相互作用,作为 A 结构域相对于 HAD 部分运动的铰链区。



























 京公网安备 11010802027423号
京公网安备 11010802027423号