当前位置:
X-MOL 学术
›
J. Food Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A high‐fructose diet in rats induces systemic iron deficiency and hepatic iron overload by an inflammation mechanism
Journal of Food Biochemistry ( IF 4 ) Pub Date : 2020-12-02 , DOI: 10.1111/jfbc.13578 Chao Wang 1, 2 , Xing Wang 2 , Guangyao Song 3 , Hanying Xing 2 , Linquan Yang 1 , Kang Han 1 , Yan-Zhong Chang 1
Journal of Food Biochemistry ( IF 4 ) Pub Date : 2020-12-02 , DOI: 10.1111/jfbc.13578 Chao Wang 1, 2 , Xing Wang 2 , Guangyao Song 3 , Hanying Xing 2 , Linquan Yang 1 , Kang Han 1 , Yan-Zhong Chang 1
Affiliation
|
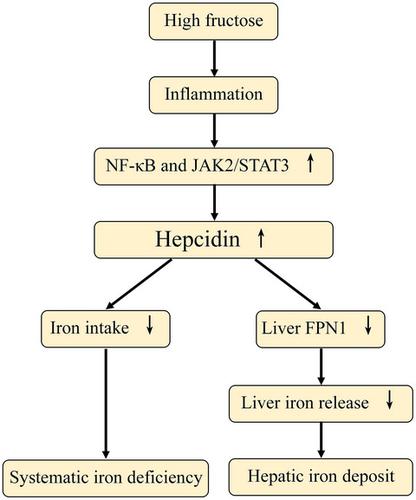
Nonalcoholic fatty liver disease (NAFLD) correlates with the high intake of fructose‐rich soft drinks. Both inflammation and dysregulated iron metabolism are pathogenic factors in the development of NAFLD. The present investigation assessed the effects of a high‐fructose diet (HF diet) on inflammation and iron metabolism. In this study, rats were fed a control or HF diet for 4, 8, or 12 weeks, after which insulin resistance, transaminases levels, serum and liver lipid profiles, inflammatory factors, and iron metabolism‐related molecules were evaluated. The activities of the hepatic inflammation‐associated pathways, IKKβ/NF‐κB, and JAK2/STAT3, were detected by western blot. Result showed that the HF diet‐fed animals developed a time‐dependent serum lipid increase and hepatic lipid accumulation as well as insulin resistance. Serum iron (SI), serum ferritin (SF), and transferrin saturation (TS) decreased while total iron‐binding capacity (TIBC) and serum transferrin (s‐TF) increased at 8 and 12 weeks in the HF diet group. The HF diet led to increased transaminases levels at 8 and 12 weeks, and iron deposition was observed in the liver, accompanied by an upregulation of ferritin light chain (FTL), hepcidin (HEPC), transferrin (TF), transferrin receptor 1 (TfR1), iron regulatory protein 1 (IRP1), hemojuvelin (HJV), and divalent metal transporter 1 (DMT1). Moreover, ferroportin (FPN1) levels were downregulated, as expected from the increased HEPC. A progressive inflammation phenotype was apparent, with increased inflammatory factors, MDA, IL‐1β, IL‐6, and TNF‐α, in the serum and liver tissue. Concomitantly, the hepatic IKKβ/NF‐κB and JAK2/STAT3 pathways were activated. In summary, we verified that HF diet induces systemic iron deficiency and hepatic iron accumulation, likely due to the activation of inflammation via the NF‐κB and JAK2/STAT3 pathways.
中文翻译:

大鼠高果糖饮食会通过炎症机制诱发系统性铁缺乏和肝铁超负荷
非酒精性脂肪性肝病(NAFLD)与富含果糖的软饮料的摄入量有关。炎症和铁代谢失调都是NAFLD发展的致病因素。本研究评估了高果糖饮食(HF饮食)对炎症和铁代谢的影响。在这项研究中,大鼠接受了4,8或12周的对照饮食或HF饮食,之后评估了胰岛素抵抗,转氨酶水平,血清和肝脂质谱,炎性因子以及铁代谢相关分子。免疫印迹检测了肝炎症相关通路IKKβ/NF-κB和JAK2 / STAT3的活性。结果表明,高脂饮食喂养的动物出现了随时间变化的血清脂质升高,肝脂质蓄积以及胰岛素抵抗。血清铁(SI)HF饮食组在8和12周时血清铁蛋白(SF)和转铁蛋白饱和度(TS)降低,而总铁结合能力(TIBC)和血清转铁蛋白(s-TF)升高。HF饮食导致8和12周转氨酶水平升高,并且在肝脏中观察到铁沉积,并伴有铁蛋白轻链(FTL),铁调素(HEPC),转铁蛋白(TF),转铁蛋白受体1(TfR1)的上调),铁调节蛋白1(IRP1),血丝素(HJV)和二价金属转运蛋白1(DMT1)。此外,铁蛋白转运蛋白(FPN1)水平下调,正如HEPC升高所预期的那样。血清和肝组织中明显出现了进行性炎症表型,炎症因子,MDA,IL-1β,IL-6和TNF-α升高。同时,肝的IKKβ/NF-κB和JAK2 / STAT3途径被激活。综上所述,
更新日期:2021-01-19
中文翻译:

大鼠高果糖饮食会通过炎症机制诱发系统性铁缺乏和肝铁超负荷
非酒精性脂肪性肝病(NAFLD)与富含果糖的软饮料的摄入量有关。炎症和铁代谢失调都是NAFLD发展的致病因素。本研究评估了高果糖饮食(HF饮食)对炎症和铁代谢的影响。在这项研究中,大鼠接受了4,8或12周的对照饮食或HF饮食,之后评估了胰岛素抵抗,转氨酶水平,血清和肝脂质谱,炎性因子以及铁代谢相关分子。免疫印迹检测了肝炎症相关通路IKKβ/NF-κB和JAK2 / STAT3的活性。结果表明,高脂饮食喂养的动物出现了随时间变化的血清脂质升高,肝脂质蓄积以及胰岛素抵抗。血清铁(SI)HF饮食组在8和12周时血清铁蛋白(SF)和转铁蛋白饱和度(TS)降低,而总铁结合能力(TIBC)和血清转铁蛋白(s-TF)升高。HF饮食导致8和12周转氨酶水平升高,并且在肝脏中观察到铁沉积,并伴有铁蛋白轻链(FTL),铁调素(HEPC),转铁蛋白(TF),转铁蛋白受体1(TfR1)的上调),铁调节蛋白1(IRP1),血丝素(HJV)和二价金属转运蛋白1(DMT1)。此外,铁蛋白转运蛋白(FPN1)水平下调,正如HEPC升高所预期的那样。血清和肝组织中明显出现了进行性炎症表型,炎症因子,MDA,IL-1β,IL-6和TNF-α升高。同时,肝的IKKβ/NF-κB和JAK2 / STAT3途径被激活。综上所述,



























 京公网安备 11010802027423号
京公网安备 11010802027423号