Molecular Catalysis ( IF 4.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.mcat.2020.111318 Chunhui Liu , Shi-Jun Li , Peilin Han , Ling-Bo Qu , Yu Lan
|
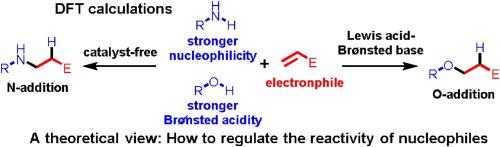
Regulating the reactivity of nucleophiles is important in synthetic chemistry. As a weaker nucleophile is also a stronger Brønsted acid, it can be selectively deprotonated by a Brønsted base to give an anionic species that shows higher reactivity. Following this idea, the reactivity of nucleophiles can be controlled by using an appropriate Lewis acid/Brønsted base pair catalyst. Cuprous/aminolithium catalyzed hydrofunctionalization of acrylonitrile with an alcohol and an amine were chosen as model reactions. As the acidity of the alcohol is significantly higher than that of amine, the former is more easily deprotonated. Therefore, an alcohol shows higher reactivity than an amine in the presence of a Lewis acid/Brønsted base pair catalyst, reversing the selectivity trend.
中文翻译:

如何通过使用路易斯酸/布朗斯台德碱对催化剂逆转亲核加成的化学选择性:理论观点
调节亲核试剂的反应性在合成化学中很重要。由于较弱的亲核试剂也是较强的布朗斯台德酸,因此可以通过布朗斯台德碱选择性地使其质子化,从而得到显示更高反应性的阴离子物质。按照这个想法,可以通过使用适当的路易斯酸/布朗斯台德碱对催化剂来控制亲核试剂的反应性。选择用醇和胺的亚铜/氨基锂催化的丙烯腈加氢官能化作为模型反应。由于醇的酸度明显高于胺的酸度,因此前者更容易去质子化。因此,在路易斯酸/布朗斯台德碱对催化剂的存在下,醇比胺具有更高的反应活性,从而逆转了选择性趋势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号