当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Mononuclear and High-Spin Tetrahedral TiII Complex
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.inorgchem.0c02586 Anders Reinholdt 1 , Daniel Pividori 2 , Alexander L. Laughlin 3 , Ida M. DiMucci 3 , Samantha N. MacMillan 3 , Mehrafshan G. Jafari 1 , Michael R. Gau 1 , Patrick J. Carroll 1 , J. Krzystek 4 , Andrew Ozarowski 4 , Joshua Telser 5 , Kyle M. Lancaster 3 , Karsten Meyer 2 , Daniel J. Mindiola 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-12-01 , DOI: 10.1021/acs.inorgchem.0c02586 Anders Reinholdt 1 , Daniel Pividori 2 , Alexander L. Laughlin 3 , Ida M. DiMucci 3 , Samantha N. MacMillan 3 , Mehrafshan G. Jafari 1 , Michael R. Gau 1 , Patrick J. Carroll 1 , J. Krzystek 4 , Andrew Ozarowski 4 , Joshua Telser 5 , Kyle M. Lancaster 3 , Karsten Meyer 2 , Daniel J. Mindiola 1
Affiliation
|
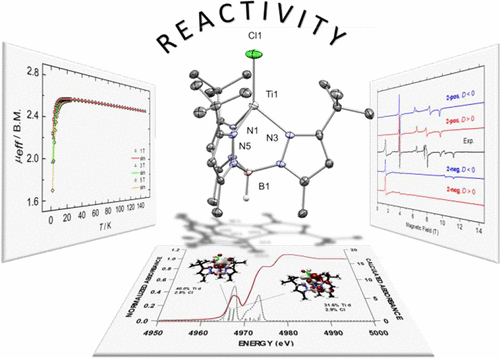
A high-spin, mononuclear TiII complex, [(TptBu,Me)TiCl] [TptBu,Me– = hydridotris(3-tert-butyl-5-methylpyrazol-1-yl)borate], confined to a tetrahedral ligand-field environment, has been prepared by reduction of the precursor [(TptBu,Me)TiCl2] with KC8. Complex [(TptBu,Me)TiCl] has a 3A2 ground state (assuming C3v symmetry based on structural studies), established via a combination of high-frequency and -field electron paramagnetic resonance (HFEPR) spectroscopy, solution and solid-state magnetic studies, Ti K-edge X-ray absorption spectroscopy (XAS), and both density functional theory and ab initio (complete-active-space self-consistent-field, CASSCF) calculations. The formally and physically defined TiII complex readily binds tetrahydrofuran (THF) to form the paramagnetic adduct [(TptBu,Me)TiCl(THF)], which is impervious to N2 binding. However, in the absence of THF, the TiII complex captures N2 to produce the diamagnetic complex [(TptBu,Me)TiCl]2(η1,η1;μ2-N2), with a linear Ti═N═N═Ti topology, established by single-crystal X-ray diffraction. The N2 complex was characterized using XAS as well as IR and Raman spectroscopies, thus establishing this complex to possess two TiIII centers covalently bridged by an N22– unit. A π acid such as CNAd (Ad = 1-adamantyl) coordinates to [(TptBu,Me)TiCl] without inducing spin pairing of the d electrons, thereby forming a unique high-spin and five-coordinate TiII complex, namely, [(TptBu,Me)TiCl(CNAd)]. The reducing power of the coordinatively unsaturated TiII-containing [(ΤptBu,Me)TiCl] species, quantified by electrochemistry, provides access to a family of mononuclear TiIV complexes of the type [(TptBu,Me)Ti═E(Cl)] (with E2– = NSiMe3, N2CPh2, O, and NH) by virtue of atom- or group-transfer reactions using various small molecules such as N3SiMe3, N2CPh2, N2O, and the bicyclic amine 2,3:5,6-dibenzo-7-azabicyclo[2.2.1]hepta-2,5-diene.
中文翻译:

单核高旋转四面体Ti II复合物
高自旋,单核Ti II复合物[[(Tp t Bu,Me)TiCl] [Tp t Bu,Me– =氢化三(3-叔丁基-5-甲基吡唑-1-基)硼酸盐],通过用KC 8还原前体[(Tp t Bu,Me)TiCl 2 ]制备了四面体配体场环境。络合物[(Tp t Bu,Me)TiCl]具有3 A 2基态(根据结构研究假设C 3 v对称),通过结合了高频和场电子顺磁共振(HFEPR)光谱,溶液和固态磁研究,Ti K边缘X射线吸收光谱(XAS)以及密度泛函理论和从头算(完全活性空间自洽字段(CASSCF)计算。形式上和物理上定义的Ti II复合物很容易与四氢呋喃(THF)结合形成顺磁性加合物[(Tp t Bu,Me)TiCl(THF)],它不渗透N 2。然而,在不存在的THF中,在Ti II复杂捕获Ñ 2,以产生反磁性络合物[(TP吨卜中,Me)的TiCl] 2(η 1,η 1;μ 2 -N 2),用线性Ti═N═N═Ti拓扑,通过单晶X射线衍射确定。使用XAS以及IR和拉曼光谱对N 2络合物进行了表征,从而确定了该络合物具有两个由N 2 2-单元共价桥接的Ti III中心。诸如CNAd(Ad = 1-金刚烷基)之类的π酸与[(Tp t Bu,Me)TiCl]配位而不会引起d电子的自旋配对,从而形成独特的高自旋和五坐标Ti II配合物,即,[(Tp t Bu,Me)TiCl(CNAd)]。含不饱和Ti II的[(Τpt Bu,Me)TiCl]种类,通过电化学定量,可以访问[[Tp t Bu,Me)Ti═E(Cl)]类型的单核Ti IV配合物族(其中E 2– = NSiMe 3, N 2 CPh 2,O和NH)借助原子转移或基团转移反应,使用各种小分子,例如N 3 SiMe 3,N 2 CPh 2,N 2 O和双环胺2,3:5, 6-二苯并-7-氮杂双环[2.2.1]庚-2,5-二烯。
更新日期:2020-12-21
中文翻译:

单核高旋转四面体Ti II复合物
高自旋,单核Ti II复合物[[(Tp t Bu,Me)TiCl] [Tp t Bu,Me– =氢化三(3-叔丁基-5-甲基吡唑-1-基)硼酸盐],通过用KC 8还原前体[(Tp t Bu,Me)TiCl 2 ]制备了四面体配体场环境。络合物[(Tp t Bu,Me)TiCl]具有3 A 2基态(根据结构研究假设C 3 v对称),通过结合了高频和场电子顺磁共振(HFEPR)光谱,溶液和固态磁研究,Ti K边缘X射线吸收光谱(XAS)以及密度泛函理论和从头算(完全活性空间自洽字段(CASSCF)计算。形式上和物理上定义的Ti II复合物很容易与四氢呋喃(THF)结合形成顺磁性加合物[(Tp t Bu,Me)TiCl(THF)],它不渗透N 2。然而,在不存在的THF中,在Ti II复杂捕获Ñ 2,以产生反磁性络合物[(TP吨卜中,Me)的TiCl] 2(η 1,η 1;μ 2 -N 2),用线性Ti═N═N═Ti拓扑,通过单晶X射线衍射确定。使用XAS以及IR和拉曼光谱对N 2络合物进行了表征,从而确定了该络合物具有两个由N 2 2-单元共价桥接的Ti III中心。诸如CNAd(Ad = 1-金刚烷基)之类的π酸与[(Tp t Bu,Me)TiCl]配位而不会引起d电子的自旋配对,从而形成独特的高自旋和五坐标Ti II配合物,即,[(Tp t Bu,Me)TiCl(CNAd)]。含不饱和Ti II的[(Τpt Bu,Me)TiCl]种类,通过电化学定量,可以访问[[Tp t Bu,Me)Ti═E(Cl)]类型的单核Ti IV配合物族(其中E 2– = NSiMe 3, N 2 CPh 2,O和NH)借助原子转移或基团转移反应,使用各种小分子,例如N 3 SiMe 3,N 2 CPh 2,N 2 O和双环胺2,3:5, 6-二苯并-7-氮杂双环[2.2.1]庚-2,5-二烯。



























 京公网安备 11010802027423号
京公网安备 11010802027423号