当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formations of aryl or pyrrole ring via palladium‐catalyzed C?H functionalization on amido‐substituted quinones in the presence of amines or phosphines
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-12-01 , DOI: 10.1002/jccs.202000375 Cang‐Sian Li, Yi‐Ping Tseng, Ting‐Hsuan Hsu, Chiao‐Yun Chang, Fung‐E Hong
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-12-01 , DOI: 10.1002/jccs.202000375 Cang‐Sian Li, Yi‐Ping Tseng, Ting‐Hsuan Hsu, Chiao‐Yun Chang, Fung‐E Hong
|
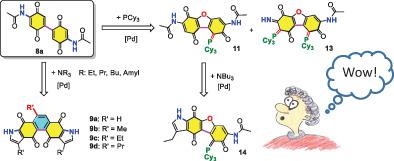
A di‐substituted quinone (5), having two amido‐groups at opposite positions, was reacted with tertiary, secondary, or primary amines via a one‐pot reaction in the presence of palladium salt. Crystal structures of some of the products, 7b‐7c, from these reactions reveal that two new pyrrole rings are formed and linked to quinone framework of 5 by taking advantage of the in situ generated alkenyl moiety, which is from the reaction of amine with palladium salt. Furthermore, the reaction of di‐amido substituted diquinone (8a) with tertiary amines led to the formations of 9a‐9d with new pyrrole and benzene rings to the moiety of 8a. Similar procedures for the reaction of 8b with tertiary amines provides an alternated route to make notable 1,4,5,8‐phenanthrenetetraone derivatives, 9e and 9f. Moreover, the reaction of diquinone (8a) with tricyclohexylphosphine led to two products, 11 and 13, with new formation of furan ring as well as PC bond. Further reaction of 11 with NBu3 yielded 14 with newly formed pyrrole ring. Crystal structures of these newly‐formed compounds revealed by single‐crystal X‐ray diffraction methods substantiate the claim.
中文翻译:

通过钯催化的C ?形成芳基或吡咯环 在胺或膦存在下在酰胺基取代的醌上进行H官能化
在钯盐存在下,通过一锅法反应,在相对位置具有两个酰胺基的双取代醌(5)与叔,仲或伯胺反应。这些反应的某些产物7b到7c的晶体结构表明,利用胺和钯的反应原位生成的烯基部分,形成了两个新的吡咯环并与5的醌骨架连接盐。此外,二氨基取代的二醌(8a)与叔胺的反应导致9a - 9d的形成,并带有新的吡咯和苯环到8a的部分。8b与叔胺反应的类似程序提供了另一种制备显着的1,4,5,8-菲四酮衍生物9e和9f的途径。此外,二醌(8a)与三环己基膦反应生成了两个产物11和13,并形成了呋喃环和PC键。11与NBu 3的进一步反应产生带有新形成的吡咯环的14。通过单晶X射线衍射方法揭示的这些新形成的化合物的晶体结构证实了这一主张。
更新日期:2020-12-01
中文翻译:

通过钯催化的C ?形成芳基或吡咯环 在胺或膦存在下在酰胺基取代的醌上进行H官能化
在钯盐存在下,通过一锅法反应,在相对位置具有两个酰胺基的双取代醌(5)与叔,仲或伯胺反应。这些反应的某些产物7b到7c的晶体结构表明,利用胺和钯的反应原位生成的烯基部分,形成了两个新的吡咯环并与5的醌骨架连接盐。此外,二氨基取代的二醌(8a)与叔胺的反应导致9a - 9d的形成,并带有新的吡咯和苯环到8a的部分。8b与叔胺反应的类似程序提供了另一种制备显着的1,4,5,8-菲四酮衍生物9e和9f的途径。此外,二醌(8a)与三环己基膦反应生成了两个产物11和13,并形成了呋喃环和PC键。11与NBu 3的进一步反应产生带有新形成的吡咯环的14。通过单晶X射线衍射方法揭示的这些新形成的化合物的晶体结构证实了这一主张。



























 京公网安备 11010802027423号
京公网安备 11010802027423号