当前位置:
X-MOL 学术
›
Int. J. Energy Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Band gap engineering of ZnO by amino acid capping for optoelectronic and energy applications
International Journal of Energy Research ( IF 4.6 ) Pub Date : 2020-11-30 , DOI: 10.1002/er.6214 Jositta Sherine 1 , Emayavaramban Indubala 2 , Harsh Anish 1 , Harinipriya Seshadri 3
International Journal of Energy Research ( IF 4.6 ) Pub Date : 2020-11-30 , DOI: 10.1002/er.6214 Jositta Sherine 1 , Emayavaramban Indubala 2 , Harsh Anish 1 , Harinipriya Seshadri 3
Affiliation
|
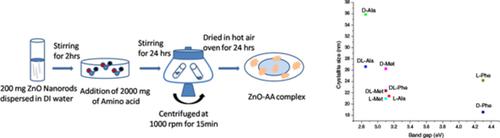
ZnO nanorods were capped with aliphatic, aromatic, and heterocyclic amino acids and the effect of capping on morphology, structural, optical, electrochemical, and electrical properties were studied in detail. The formation of coordination complexes between amino acids and ZnO were confirmed by XRD, XPS, and FTIR. The binding energy difference; ∆E = 23 eV explicitly indicates the nanometer range of the complexes formed. The high Iuv/Ivis seen in PL, for all ZnO‐AA complexes except ZnO‐L‐Phe and ZnO‐D‐Phe indicate the crystallinity and minimal defect in the complexes. The band gap of ZnO‐D‐Ala and ZnO‐DL‐Ala reduced by 0.15 eV compared to ZnO NRs (3.0 eV). This reduction in band gap is attributed to the (a) electronic percolation between C=O group of amino acid and Zn in ZnO and (b) increase in crystallite size due to amino acid capping. The free energy of activation for electronic conductivity, in case of ZnO‐D‐Ala and ZnO‐DL‐Ala remained identical to ZnO NRs (127 kJ) as supported by EIS analysis and Arrhenius kinetics. Out of all the aliphatic, aromatic, and heterocyclic amino acids capping on ZnO NRs, the results indicate that ZnO‐D‐Ala and ZnO‐DL‐Ala complexes possess lower band gap and can be used as a viable n‐type semiconductor material in optoelectronic applications such as energy harvesting devices like solar cells.
中文翻译:

通过氨基酸封端对ZnO进行带隙工程,用于光电和能源应用
ZnO纳米棒被脂肪族,芳香族和杂环氨基酸所覆盖,并且研究了封端对形态,结构,光学,电化学和电学性质的影响。XRD,XPS和FTIR证实了氨基酸与ZnO之间配位络合物的形成。结合能差;∆ E = 23 eV明确表示形成的络合物的纳米范围。高I uv / I vis从PL中可以看出,除ZnO-L-Phe和ZnO-D-Phe以外的所有ZnO-AA复合物均表明该复合物中的结晶度和最小缺陷。与ZnO NRs(3.0 eV)相比,ZnO-D-Ala和ZnO-DL-Ala的带隙降低了0.15 eV。带隙的减小归因于(a)ZnO中氨基酸的C = O组与Zn之间的电子渗透以及(b)由于氨基酸封端而增加了微晶尺寸。在EIS分析和Arrhenius动力学的支持下,在ZnO-D-Ala和ZnO-DL-Ala的情况下,电导率激活的自由能仍与ZnO NRs(127 kJ)相同。在所有覆盖ZnO NR的脂族,芳族和杂环氨基酸中,
更新日期:2020-11-30
中文翻译:

通过氨基酸封端对ZnO进行带隙工程,用于光电和能源应用
ZnO纳米棒被脂肪族,芳香族和杂环氨基酸所覆盖,并且研究了封端对形态,结构,光学,电化学和电学性质的影响。XRD,XPS和FTIR证实了氨基酸与ZnO之间配位络合物的形成。结合能差;∆ E = 23 eV明确表示形成的络合物的纳米范围。高I uv / I vis从PL中可以看出,除ZnO-L-Phe和ZnO-D-Phe以外的所有ZnO-AA复合物均表明该复合物中的结晶度和最小缺陷。与ZnO NRs(3.0 eV)相比,ZnO-D-Ala和ZnO-DL-Ala的带隙降低了0.15 eV。带隙的减小归因于(a)ZnO中氨基酸的C = O组与Zn之间的电子渗透以及(b)由于氨基酸封端而增加了微晶尺寸。在EIS分析和Arrhenius动力学的支持下,在ZnO-D-Ala和ZnO-DL-Ala的情况下,电导率激活的自由能仍与ZnO NRs(127 kJ)相同。在所有覆盖ZnO NR的脂族,芳族和杂环氨基酸中,


























 京公网安备 11010802027423号
京公网安备 11010802027423号