Chemosphere ( IF 8.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.chemosphere.2020.129104 Kohei Tokunaga , Yoshio Takahashi , Kazuya Tanaka , Naofumi Kozai
|
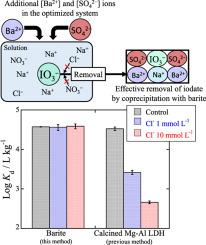
Radioactive iodine (129I) is of great concern owing to its high mobility in the environment and long-term radiotoxicity. However, there is a lack of effective techniques for removing iodate (IO3−) from aqueous solution. This study aims to develop a new technique for removing radioactive iodate from contaminated solution by using barite (BaSO4). We examined the coprecipitation mechanism of iodate by barite at the molecular level to determine the optimum conditions for iodate removal. Results showed that iodate was effectively removed from the aqueous solution by coprecipitation even in the presence of competitive anions. Based on comparison of our method with previous techniques, the iodate removal efficiency by barite was determined to be about two orders of magnitude greater than that by hydrotalcite-like layered double hydroxide at 10 mmol L−1 Cl−. Extended X-ray absorption fine structure analysis indicated that the incorporated iodate was strongly bound to the crystal lattice of barite by substituting the sulfate site in the structure when the iodine concentration was low. The charge compensation problem from the IO3− substitution in the SO42− site was achieved by the substitution of Na+−IO3− pairs at the nearest Ba2+ site. Given the high removal efficiency and strong binding of iodate to barite, coprecipitation with barite is a promising tool for removing radioactive iodate from various aqueous solutions contaminated with iodate.
中文翻译:

与重晶石共沉淀有效去除碘酸盐的行为和机理
放射性碘(129 I)由于其在环境中的高度移动性和长期的放射毒性而倍受关注。然而,存在缺乏用于去除碘酸盐的有效技术(IO 3 - )从水溶液。这项研究旨在开发一种使用重晶石(BaSO 4从污染物溶液中去除放射性碘酸盐的新技术)。我们在分子水平上研究了重晶石与碘酸盐的共沉淀机理,以确定去除碘酸盐的最佳条件。结果表明,即使在存在竞争性阴离子的情况下,也可以通过共沉淀有效地从水溶液中除去碘酸盐。基于我们先前的技术方法的比较,由重晶石碘酸盐去除效率经测定为约10毫摩尔大号幅度大于该两个数量由类水滑石的层状双氢氧化物-1氯- 。扩展的X射线吸收精细结构分析表明,当碘浓度低时,通过取代结构中的硫酸盐部位,掺入的碘酸盐牢固地结合在重晶石的晶格上。IO的费用补偿问题3 -取代的SO 4 2-位点通过的Na的置换来实现+ -IO 3 -在最近的Ba对2+位点。鉴于碘酸盐的高去除效率和强结合力,与重晶石的共沉淀是从各种受碘酸盐污染的水溶液中去除放射性碘酸盐的有前途的工具。


























 京公网安备 11010802027423号
京公网安备 11010802027423号