International Journal of Biological Macromolecules ( IF 8.2 ) Pub Date : 2020-11-29 , DOI: 10.1016/j.ijbiomac.2020.11.178 Chenyin Lv , Tianyan Gu , Rui Ma , Wei Yao , Yuyang Huang , Jingang Gu , Guogang Zhao
|
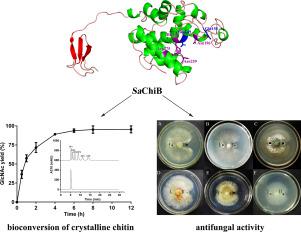
Chitinases play crucial roles in enzymatic conversion of chitin and biocontrol of phytopathogenic fungi. Herein, a chitinase of glycoside hydrolase (GH) family 19, SaChiB, was cloned from Streptomyces alfalfae ACCC 40021 and expressed in Escherichia coli BL21(DE3). The purified SaChiB displayed maximal activities at 45 °C and pH 8.0, and showed good stability up to 55 °C and in the range of pH 4.0–11.0, respectively. It exhibited substrate specificity towards chitin and chitooligosaccharides (degree of polymerization 3–6) with the endo-cleavage manner. In combination with the N-acetyl hexosaminidase SaHEX, it yielded a conversion rate of 95.2% from chitin powder to N-acetyl-D-glucosamine in 8 h and a product purity of >98.5%. Furthermore, the enzyme strongly inhibited the growth of tested pathogenic fungi. These results indicated that SaChiB has the great potential for applications in the conversion of raw chitinous waste in industries as well as the biocontrol of fungal diseases in agriculture.
中文翻译:

苜蓿链霉菌GH19几丁质酶的生化特性及其在结晶几丁质转化和生物防治中的应用
几丁质酶在几丁质的酶促转化和植物致病真菌的生物防治中起关键作用。在此,从苜蓿链霉菌ACCC 40021克隆糖苷水解酶(GH)家族的几丁质酶Sa ChiB,并在大肠杆菌BL21(DE3)中表达。纯化的Sa ChiB在45°C和pH 8.0时显示最大活性,分别在高达55°C和pH 4.0–11.0的范围内显示出良好的稳定性。它以内切方式表现出对几丁质和壳寡糖的底物特异性(聚合度3–6)。与N-乙酰基己糖胺酶Sa组合十六烷值,在8小时内从几丁质粉末到N-乙酰基-D-葡糖胺的转化率达到95.2%,产品纯度> 98.5%。此外,该酶强烈抑制了测试病原真菌的生长。这些结果表明,Sa ChiB在工业中原始几丁质废物的转化以及农业真菌病的生物防治中具有巨大的应用潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号