Chemosphere ( IF 8.8 ) Pub Date : 2020-11-30 , DOI: 10.1016/j.chemosphere.2020.129145 Joanna Nackiewicz , Łukasz Kołodziej , Anna Poliwoda , Małgorzata A. Broda
|
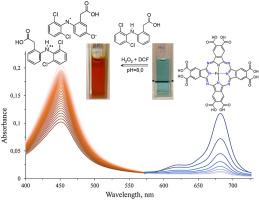
This paper presents the results of the research on the influence of catalytic activity of iron(II) octacarboxyphthalocyanines (FePcOC) on the transformation of diclofenac (DCF) which is the most popular anti-inflammatory analgesic. Diclofenac poses a serious threat to the natural environment. The paper demonstrates that diclofenac, in the presence a monomeric form of iron octacarboxyphthalocyanine and hydroxyl radicals (HO•) (from H2O2), undergoes a transformation into diclofenac-2,5-iminoquinone (DCF-2,5-IQ), causing distinct changes in the UV-Vis absorption spectrum. In the presence of iron octacarboxyphthalocyanine and H2O2, the previously colourless diclofenac solution becomes intense orange. As a result, a new band at approx. 450 nm appears in the absorption spectrum. HPLC analysis has shown that the concentration of diclofenac decreases with time. TD-DFT calculations using the CAM-B3LYP/6-31+G (d, p) method have been conducted to confirm experimental data concerning the formation of a new band at λmax = 450 nm.
中文翻译:

铁(II)八羧基酞菁存在下双氯芬酸的氧化
本文介绍了八价铁酞菁铁(IIP)催化活性对最受欢迎的消炎镇痛药双氯芬酸(DCF)转化的影响的研究结果。双氯芬酸对自然环境构成了严重威胁。本文证明双氯芬酸在八价羧基酞菁铁和羟基自由基(HO •)(来自H 2 O 2)的单体形式下,转化为双氯芬酸-2,5-亚氨基醌(DCF-2,5-IQ) ,导致UV-Vis吸收光谱发生明显变化。在八羧基酞菁铁和H 2 O 2存在下,以前无色的双氯芬酸溶液变成深橙色。结果,大约有一个新的乐队。450nm出现在吸收光谱中。HPLC分析表明双氯芬酸的浓度随时间降低。进行了使用CAM-B3LYP / 6-31 + G(d,p)方法的TD-DFT计算,以确认有关在λmax = 450 nm处形成新谱带的实验数据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号